Section Four Good Laboratory Practice Quality Assurance of

Section Four Good Laboratory Practice: Quality Assurance of Analytical Measurements

What is Good Laboratory Practice (GLP)? • The goal of GLP is to certify that every step of the analysis is valid. • Standard Operating Procedures (SOP) • Quality Assurance Unit (QAU)
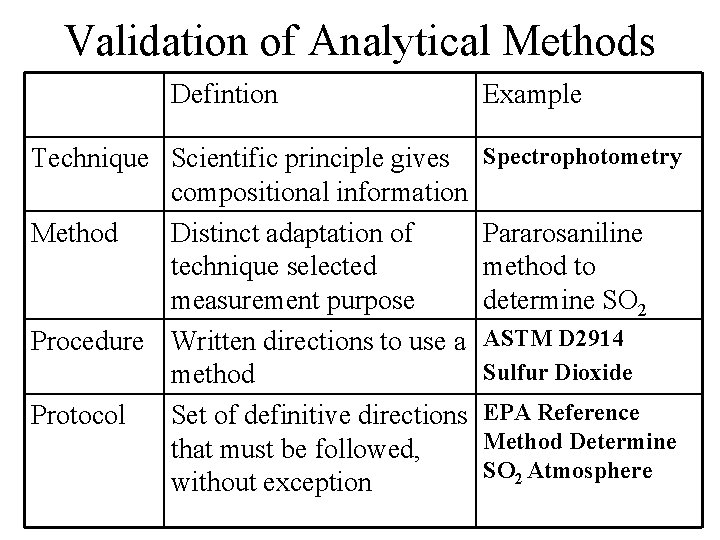
Validation of Analytical Methods Defintion Technique Scientific principle gives compositional information Method Distinct adaptation of technique selected measurement purpose Procedure Written directions to use a method Protocol Set of definitive directions that must be followed, without exception Example Spectrophotometry Pararosaniline method to determine SO 2 ASTM D 2914 Sulfur Dioxide EPA Reference Method Determine SO 2 Atmosphere
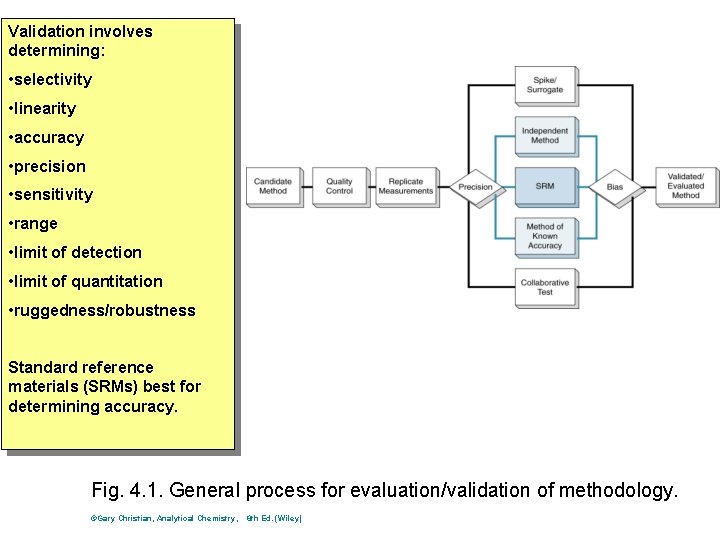
Validation involves determining: • selectivity • linearity • accuracy • precision • sensitivity • range • limit of detection • limit of quantitation • ruggedness/robustness Standard reference materials (SRMs) best for determining accuracy. Fig. 4. 1. General process for evaluation/validation of methodology. ©Gary Christian, Analytical Chemistry, 6 th Ed. (Wiley)
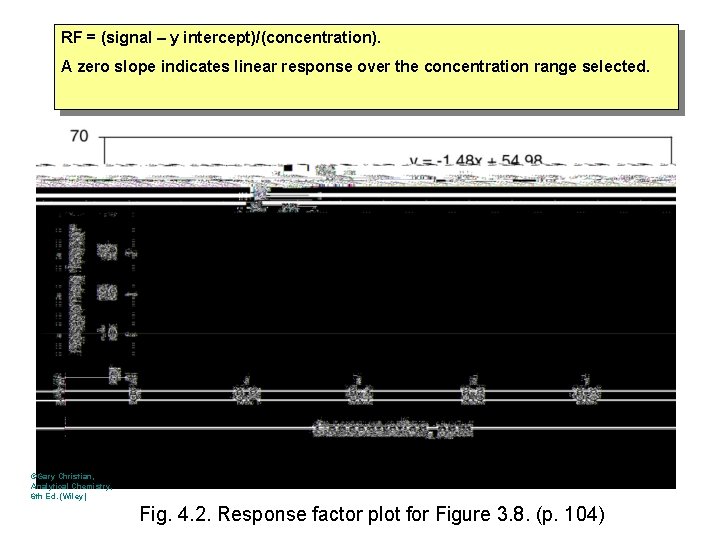
RF = (signal – y intercept)/(concentration). A zero slope indicates linear response over the concentration range selected. ©Gary Christian, Analytical Chemistry, 6 th Ed. (Wiley) Fig. 4. 2. Response factor plot for Figure 3. 8. (p. 104)
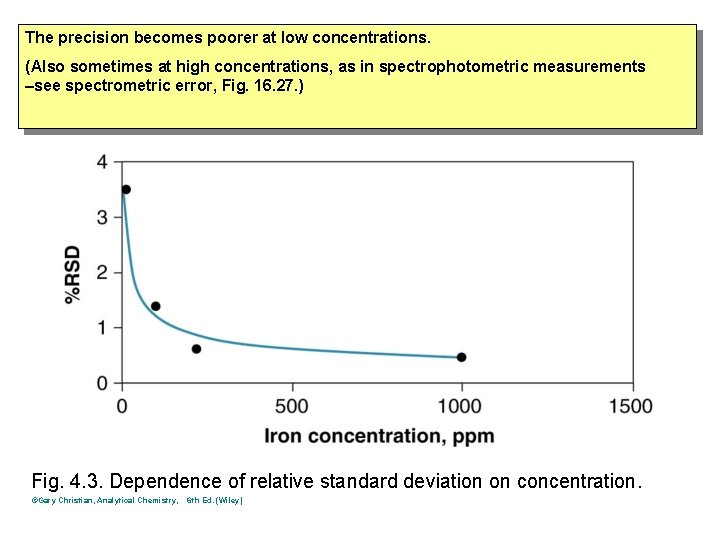
The precision becomes poorer at low concentrations. (Also sometimes at high concentrations, as in spectrophotometric measurements –see spectrometric error, Fig. 16. 27. ) Fig. 4. 3. Dependence of relative standard deviation on concentration. ©Gary Christian, Analytical Chemistry, 6 th Ed. (Wiley)
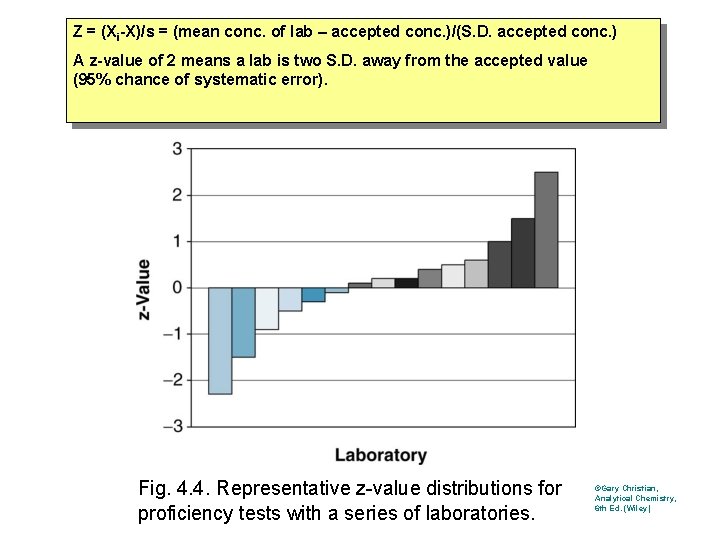
Z = (Xi-X)/s = (mean conc. of lab – accepted conc. )/(S. D. accepted conc. ) A z-value of 2 means a lab is two S. D. away from the accepted value (95% chance of systematic error). Fig. 4. 4. Representative z-value distributions for proficiency tests with a series of laboratories. ©Gary Christian, Analytical Chemistry, 6 th Ed. (Wiley)
- Slides: 7