Section 7 Physical and Chemical Oceanography Part I





- Slides: 12

Section 7. Physical and Chemical Oceanography Part I: Chemical Composition Content 1. Factors affecting the chemical composition of seawater. 2. Layering and mixing in the oceans. Learning Outcomes a) demonstrate an understanding of the effects of volcanic activity, runoff and atmospheric dissolution on the chemical composition of sea water b) outline the effects of evaporation and precipitation on salinity c) describe how temperature and salinity gradients form in water columns to produce ocean layers (including the surface layer, thermocline and deep ocean), and how subsequent mixing of these layers may occur d) demonstrate an understanding of the physical and biological reasons for the variability of the concentration of dissolved oxygen

Physical and Chemical Oceanography SECTION 7 PART I: CHEMICAL COMPOSITION Excellent Site for Review!

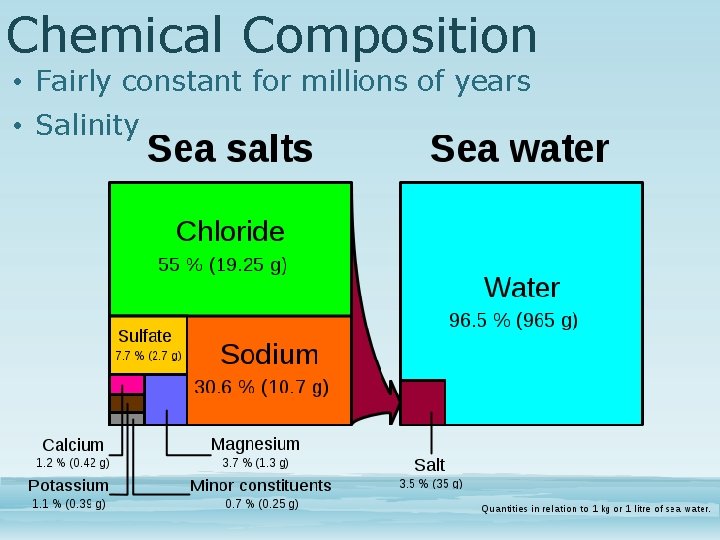
Chemical Composition • Fairly constant for millions of years • Salinity

Salinity • salt concentration in parts per thousand (ppt) • ocean average: 35 ppt (35%) • Variance: • Sea diluted with freshwater by: • River • Melting glaciers

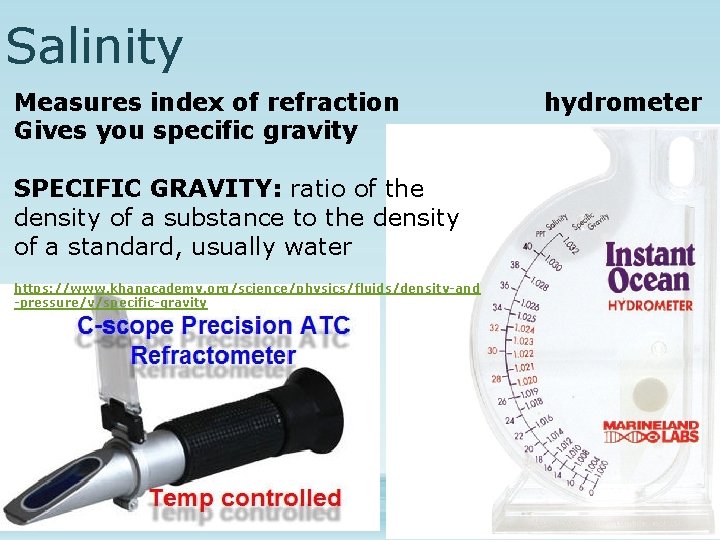
Salinity Measures index of refraction Gives you specific gravity SPECIFIC GRAVITY: ratio of the density of a substance to the density of a standard, usually water https: //www. khanacademy. org/science/physics/fluids/density-and -pressure/v/specific-gravity hydrometer

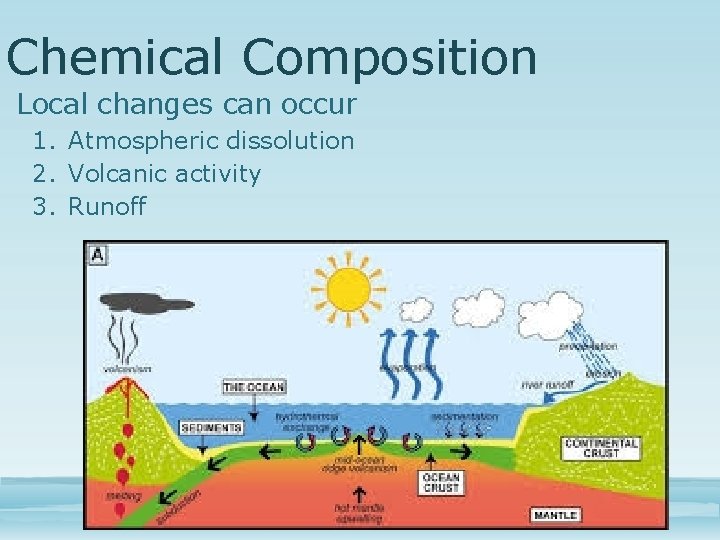
Chemical Composition Local changes can occur 1. Atmospheric dissolution 2. Volcanic activity 3. Runoff

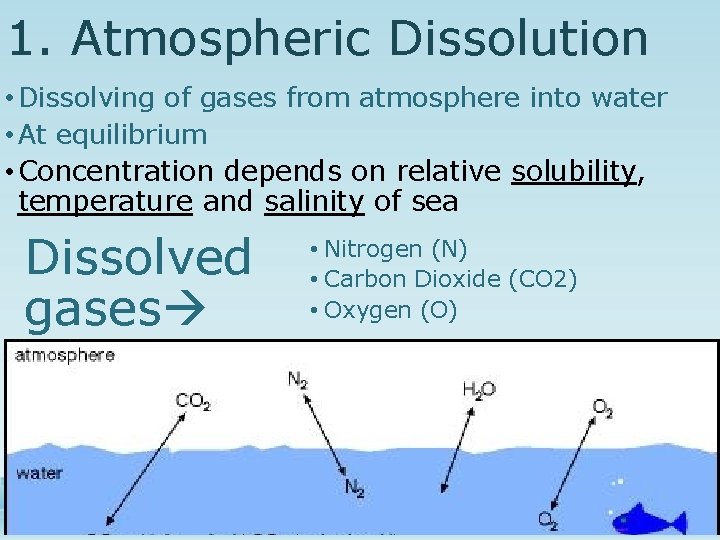
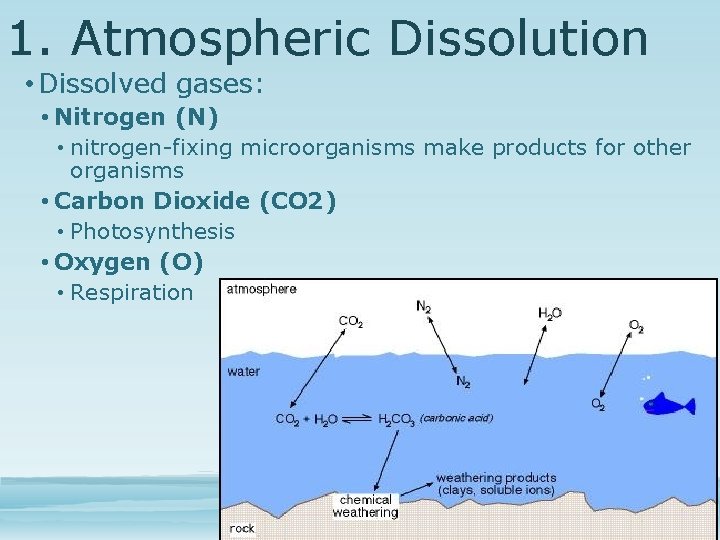
1. Atmospheric Dissolution • Dissolving of gases from atmosphere into water • At equilibrium • Concentration depends on relative solubility, temperature and salinity of sea Dissolved gases • Nitrogen (N) • Carbon Dioxide (CO 2) • Oxygen (O)

1. Atmospheric Dissolution • Dissolved gases: • Nitrogen (N) • nitrogen-fixing microorganisms make products for other organisms • Carbon Dioxide (CO 2) • Photosynthesis • Oxygen (O) • Respiration

2. Volcanic Activity ABOVEGROUND • Gases: CO 2, SO 2, H 2 S, HCl • & ash particulates • dissolve in atmospheric H 2 O • enter sea by precipitation • Changes local ion concentrations

2. Volcanic Activity UNDERWATER • Submerged volcanoes at plate boundaries emit gases • Gases: CO 2, SO 2, H 2 S, HCl • Major source of chloride ions in sea

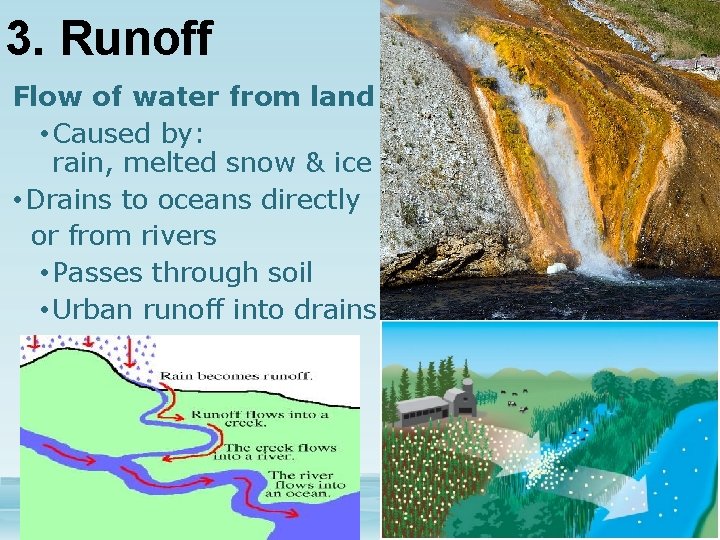
3. Runoff Flow of water from land • Caused by: rain, melted snow & ice • Drains to oceans directly or from rivers • Passes through soil • Urban runoff into drains

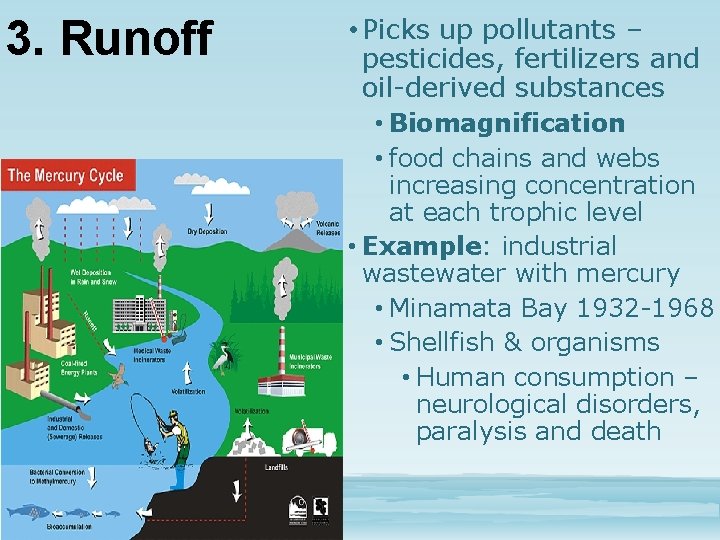
3. Runoff • Picks up pollutants – pesticides, fertilizers and oil-derived substances • Biomagnification • food chains and webs increasing concentration at each trophic level • Example: industrial wastewater with mercury • Minamata Bay 1932 -1968 • Shellfish & organisms • Human consumption – neurological disorders, paralysis and death