Section 7 1 Evidence for a Chemical Reaction
















- Slides: 25

Section 7. 1 Evidence for a Chemical Reaction Objectives 1. To learn the signals or evidences that show a chemical reaction may have occurred

Section 7. 1 Evidence for a Chemical Reaction

Section 7. 1 Evidence for a Chemical Reaction 1. Whoosh 2. Can You Identify any Evidences of a Chemical Reaction?

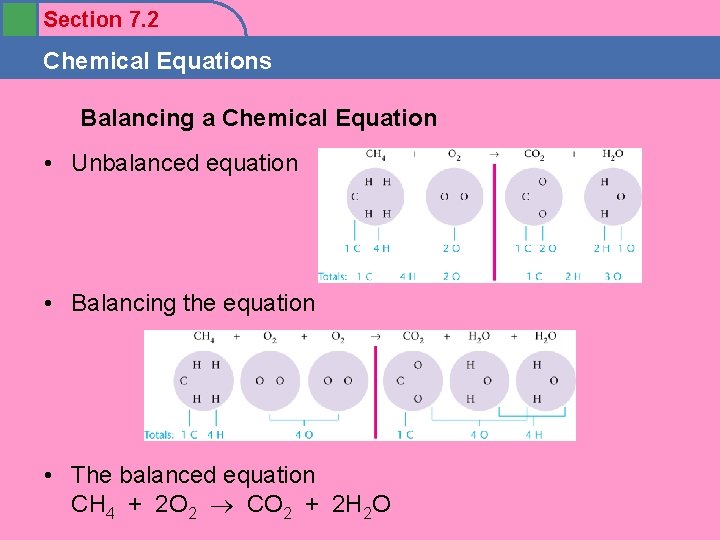
Section 7. 2 Chemical Equations Objectives 1. To learn to identify the characteristics of a chemical reaction 2. To learn the information given by a chemical equation

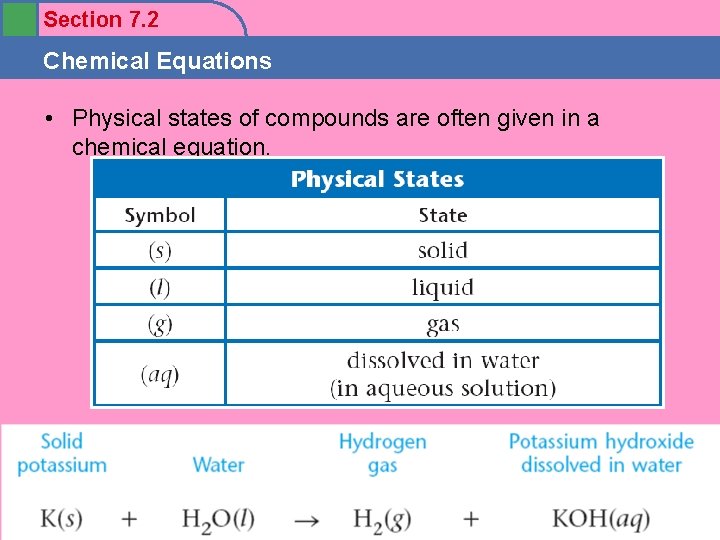
Section 7. 2 Chemical Equations • Physical states of compounds are often given in a chemical equation.

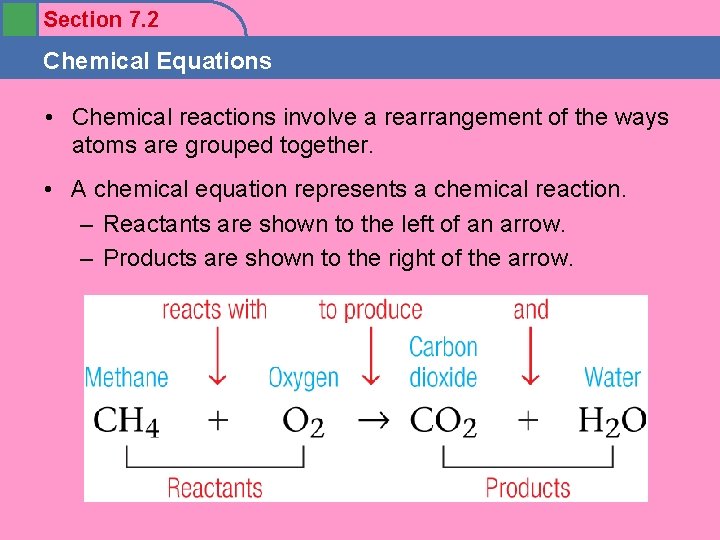
Section 7. 2 Chemical Equations • Chemical reactions involve a rearrangement of the ways atoms are grouped together. • A chemical equation represents a chemical reaction. – Reactants are shown to the left of an arrow. – Products are shown to the right of the arrow.

Section 7. 2 Chemical Equations The Law of Conservation of Mass states that mass is neither created nor destroyed during a chemical reaction. Plop, Fizz, Fizz…. . A chemical reaction involves the rearrangement of atoms to form new substances. (Coins)

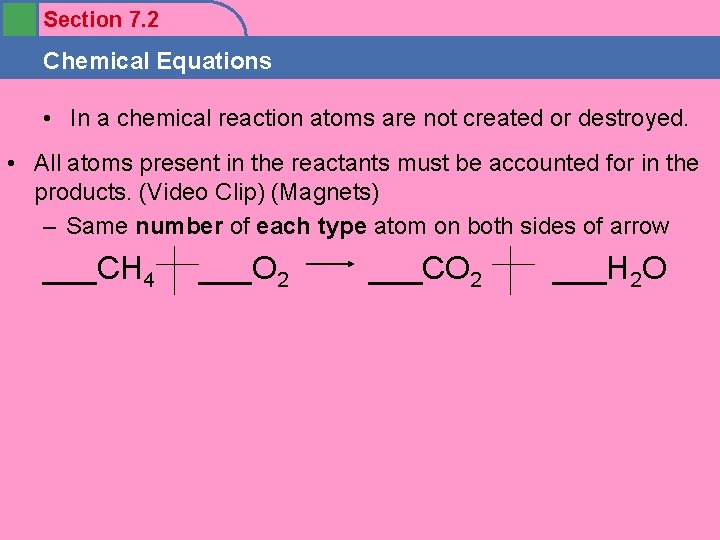
Section 7. 2 Chemical Equations • In a chemical reaction atoms are not created or destroyed. • All atoms present in the reactants must be accounted for in the products. (Video Clip) (Magnets) – Same number of each type atom on both sides of arrow ___CH 4 ___O 2 ___CO 2 ___H 2 O

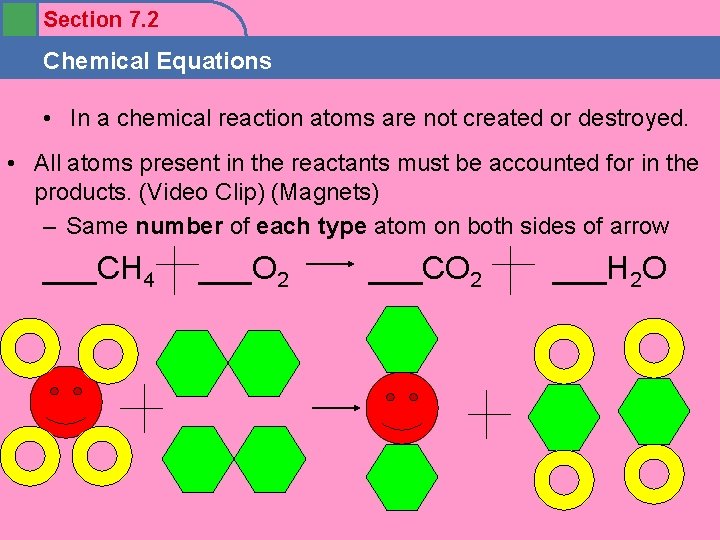
Section 7. 2 Chemical Equations • In a chemical reaction atoms are not created or destroyed. • All atoms present in the reactants must be accounted for in the products. (Video Clip) (Magnets) – Same number of each type atom on both sides of arrow ___CH 4 ___O 2 ___CO 2 ___H 2 O

Section 7. 2 Chemical Equations Objectives Review 1. To learn the signals or evidences that show a chemical reaction may have occurred 2. To learn to identify the characteristics of a chemical reaction 3. To learn the information given by a chemical equation 4. Work Session: page 234 1, 5, 6, 7, 8 5. Video of Various Chemical Rx’s Ch 7(Silver I)

Section 7. 3 Balancing Chemical Equations Objectives 1. To learn to write a balanced equation for a chemical reaction 2. Are you ready? ?

Section 7. 3 Balancing Chemical Equations

Section 7. 3 Balancing Chemical Equations • A representation of a chemical reaction: C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O • • • reactants products Reactants are only placed on the left side of the arrow, products are only placed on the right side of the arrow. The equation is balanced because all atoms present in the reactants are accounted for in the products. The balanced equation represents an overall ratio of reactants and products, not what actually “happens” during a reaction.

Section 7. 3 Balancing Chemical Equations • A chemical reaction is balanced by using a systematic approach: – Atoms (mass) are always conserved – Can only change the coefficients – Balance by trial and error starting with the most complicated molecule(s) – At the end check to be sure the equation is balanced (same numbers of all types of atoms on the reactant and product sides)

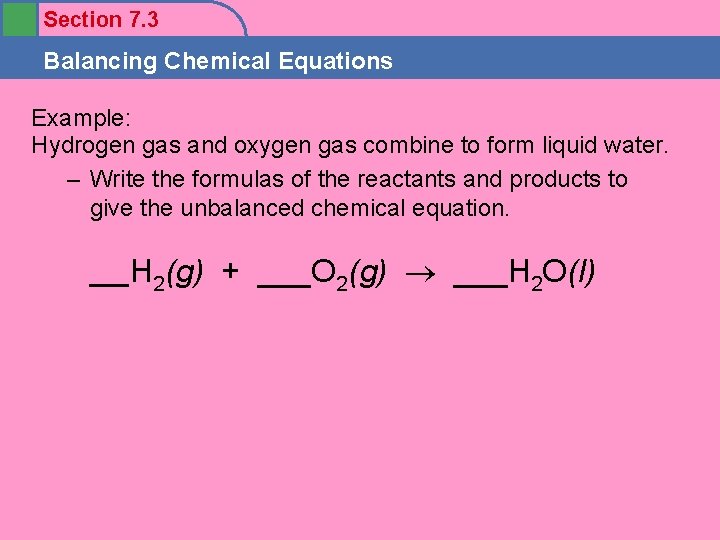
Section 7. 3 Balancing Chemical Equations Example: Hydrogen gas and oxygen gas combine to form liquid water. – Write the formulas of the reactants and products to give the unbalanced chemical equation. ___H 2(g) + ___O 2(g) ___H 2 O(l)

Section 7. 3 Balancing Chemical Equations • A chemical reaction is balanced by using a systematic approach: (Patch’s Interpretation) – Make Sure you have the equation written correctly and leave spaces in front of each compound formula. – Use a pencil and don’t be afraid of erasing! – Make sure you are NOT violating the Law of Conservation of Mass!!

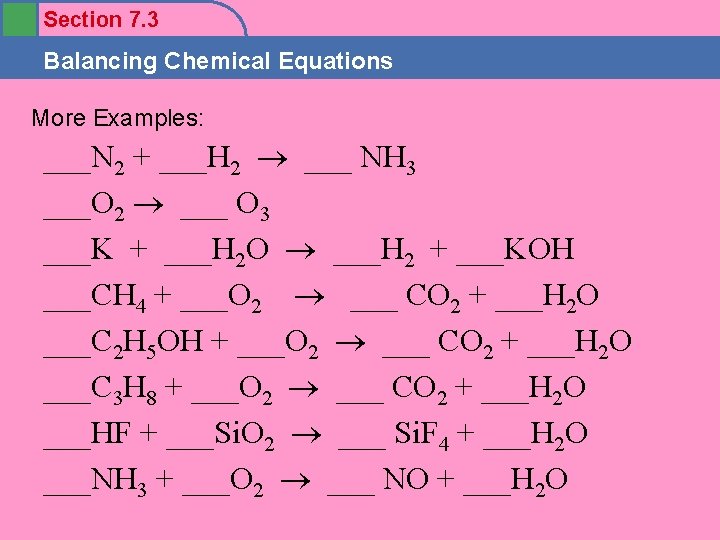
Section 7. 3 Balancing Chemical Equations More Examples: ___N 2 + ___H 2 ___ NH 3 ___O 2 ___ O 3 ___K + ___H 2 O ___H 2 + ___KOH ___CH 4 + ___O 2 ___ CO 2 + ___H 2 O ___C 2 H 5 OH + ___O 2 ___ CO 2 + ___H 2 O ___C 3 H 8 + ___O 2 ___ CO 2 + ___H 2 O ___HF + ___Si. O 2 ___ Si. F 4 + ___H 2 O ___NH 3 + ___O 2 ___ NO + ___H 2 O

Section 7. 3 Balancing Chemical Equations Independent practice: ___NH 4 NO 2 ___N 2 + ___H 2 O ___NO ___ N 2 O + ___NO 2 ___HNO 3 ___NO 2 + ___ H 2 O + ___O 2

9 Section 7. 3 Balancing Chemical Equations Concept Check Which of the following are true concerning balanced chemical equations? There may be more than one true statement. I. The number of molecules is conserved. II. The coefficients tell you how much of each substance you have. III. Atoms are neither created nor destroyed. IV. The coefficients indicate the mass ratios of the substances used. V. The sum of the coefficients on the reactant side equals the sum of the coefficients on the product side.

Section 7. 3 Balancing Chemical Equations • Let’s use all of your skills to write a reaction equation to describe the following: • Solid Carbon reacts with gaseous oxygen to form gaseous carbon dioxide. • Solid Mercury (II) oxide decomposes to produce elemental mercury metal and gaseous oxygen. • Solid zinc is added to an aqueous solution of hydrogen chloride to produce gaseous hydrogen that bubbles out of the solution and zinc chloride that remains dissolved in the water.

Section 7. 3 Balancing Chemical Equations Objectives Review 1. To learn to write a balanced equation for a chemical reaction 2. Work Session: page 234: 31, 32, 33, 34, 35, 36 Page 237: 1 -8

Section 7. 3 Balancing Chemical Equations This is the end of the required material for this chapter.

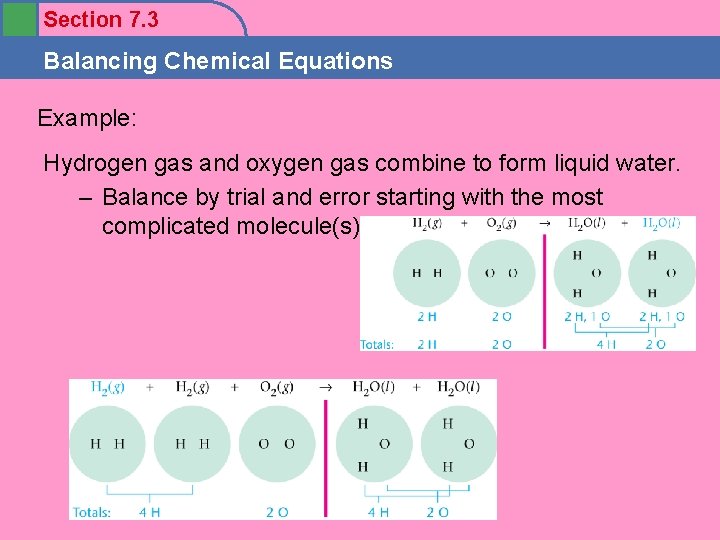
Section 7. 3 Balancing Chemical Equations Example: Hydrogen gas and oxygen gas combine to form liquid water. – Balance by trial and error starting with the most complicated molecule(s).

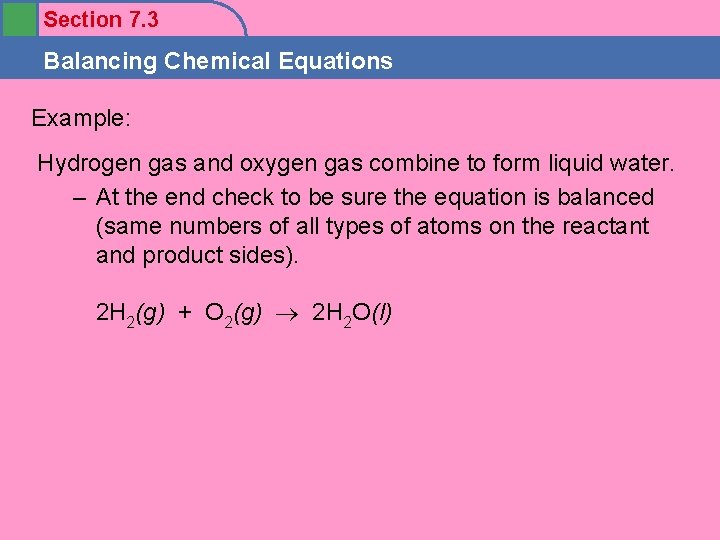
Section 7. 3 Balancing Chemical Equations Example: Hydrogen gas and oxygen gas combine to form liquid water. – At the end check to be sure the equation is balanced (same numbers of all types of atoms on the reactant and product sides). 2 H 2(g) + O 2(g) 2 H 2 O(l)

Section 7. 2 Chemical Equations Balancing a Chemical Equation • Unbalanced equation • Balancing the equation • The balanced equation CH 4 + 2 O 2 CO 2 + 2 H 2 O