Section 6 3 Acids Bases and p H

Section 6. 3 Acids, Bases, and p. H

Objectives �Compare and contrast acids and bases. �Relate the p. H of a solution to the concentration and strength of dissolved acid or base. �Identify the products of neutralization reactions.

What are Acids? �Acids make foods taste sour. �Acids give off _______ ions H+ �Some acid solutions form the maximum amount of charged ions. All acids can conduct _________ when dissolved in water Stronger acids conduct electricity better �Other acids do not ionize completely ________ will not form as many ions and therefore will not conduct electricity as well.
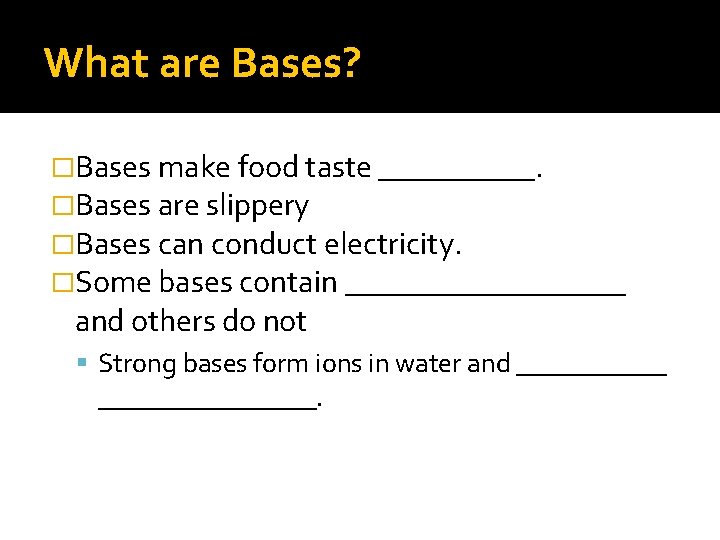
What are Bases? �Bases make food taste _____. �Bases are slippery �Bases can conduct electricity. �Some bases contain _________ and others do not Strong bases form ions in water and ________________.

What are Bases? �Other bases ___________ to form hydroxide ions. Some bases get an H+ ion from water to form ions. These bases have fewer ions, and therefore _________________ as well.

How Acidic is an Acid? �You must measure ________ ions to compare acidities. �p. H is the concentration of hydronium ions. �p. H values correspond to the ___________________. p. H is a measure of the hydronium ion concentration in a solution. p. H also indicates the solution’s hydroxide ion concentration

How Acidic is an Acid? p. H ranges from 0 to 14 ▪ Neutral solutions have a p. H of 7 ▪ Hydronium ions = hydroxide ions ▪ p. H of less than 7 are acidic ▪ Hydronium ions > hydroxide ions ▪ p. H of greater than 7 are basic ▪ Hydronium ions < hydroxide ions

Common Acids & Bases

How Acidic is an Acid? �Small differences in _______ mean larger differences in hydronium ion concentration Each p. H unit represents a factor of 10 ▪ Example: apple juice has a p. H of 3 black coffee has a p. H of 5 Apple juice is 2 units more acidic or 102 (100) times more acidic than black coffee.
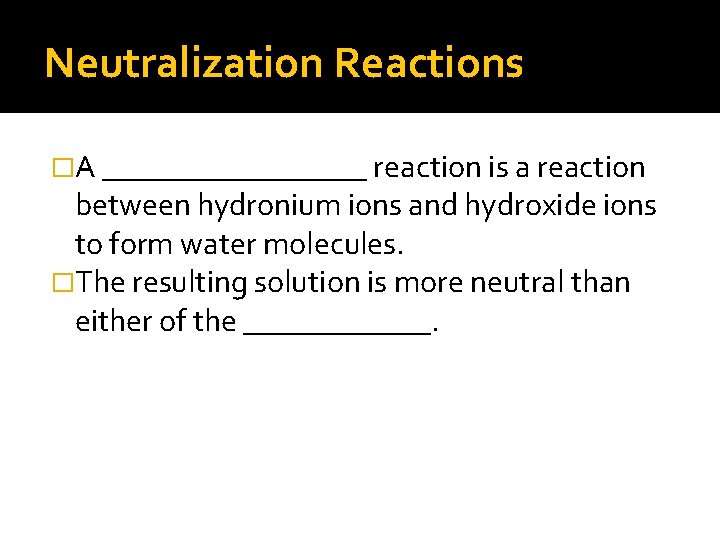
Neutralization Reactions �A _________ reaction is a reaction between hydronium ions and hydroxide ions to form water molecules. �The resulting solution is more neutral than either of the ______.
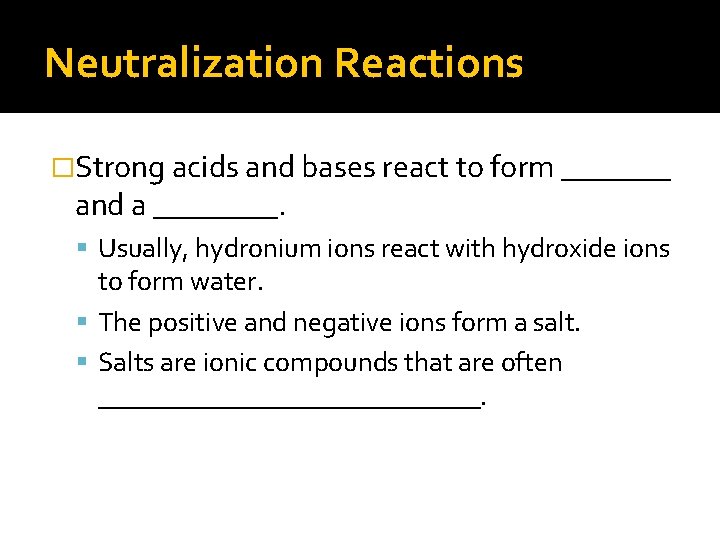
Neutralization Reactions �Strong acids and bases react to form _______ and a ____. Usually, hydronium ions react with hydroxide ions to form water. The positive and negative ions form a salt. Salts are ionic compounds that are often ______________.
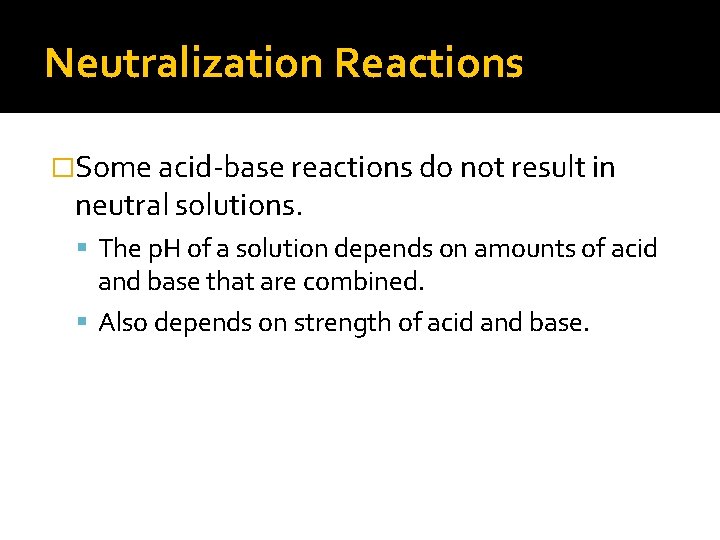
Neutralization Reactions �Some acid-base reactions do not result in neutral solutions. The p. H of a solution depends on amounts of acid and base that are combined. Also depends on strength of acid and base.

Neutralization Reactions �Avoiding dangerous reactions at home Some household products should never be combined because they reaction to form harmful substances. Example: ammonia and bleach form chloramine (poisonous) Example: vinegar and bleach form chlorine gas (poisonous) ALWAYS CHECK LABELS!!!
- Slides: 13