Section 6 2 Classification of the Elements Objectives
Section 6. 2 Classification of the Elements
Objectives n n Explain why elements in the same group have similar properties. Identify the 4 blocks of the periodic table based on electron configuration.
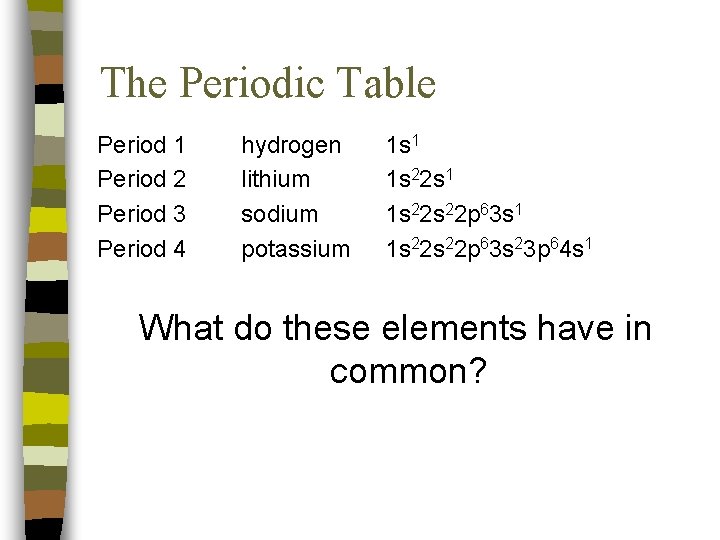
The Periodic Table Period 1 Period 2 Period 3 Period 4 hydrogen lithium sodium potassium 1 s 1 1 s 22 s 22 p 63 s 1 1 s 22 p 63 s 23 p 64 s 1 What do these elements have in common?

Classification by valence electrons Atoms of the same group have similar properties because they have the same number of valence electrons. n The number (in the group number) that accompanies the “A” designations tells you the number of valence electrons for that element. n n There is one exception. . .

Classification by valence electrons See p. 183 Fig. 7
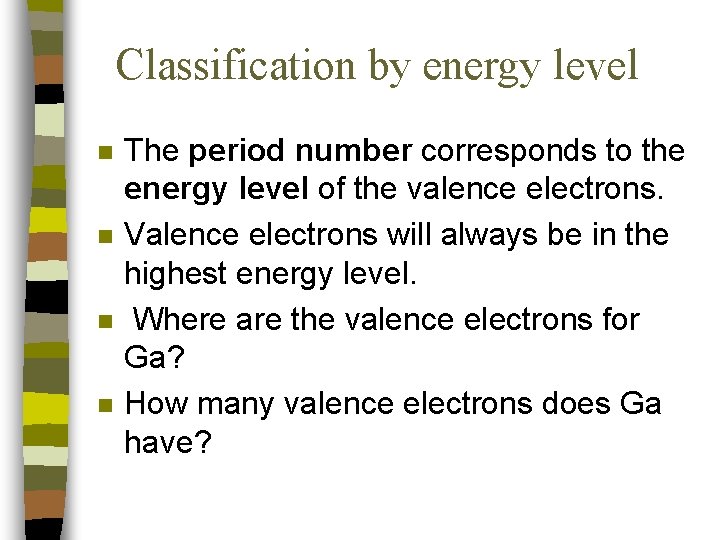
Classification by energy level n n The period number corresponds to the energy level of the valence electrons. Valence electrons will always be in the highest energy level. Where are the valence electrons for Ga? How many valence electrons does Ga have?
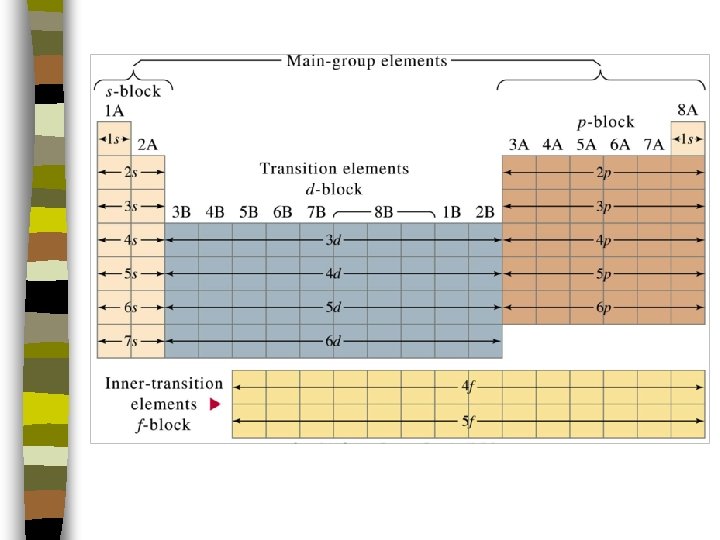
Classification by “Block” n n The Periodic Table can be viewed as arranged into blocks. Each block corresponds to the sublevel in which the outermost electrons are located.
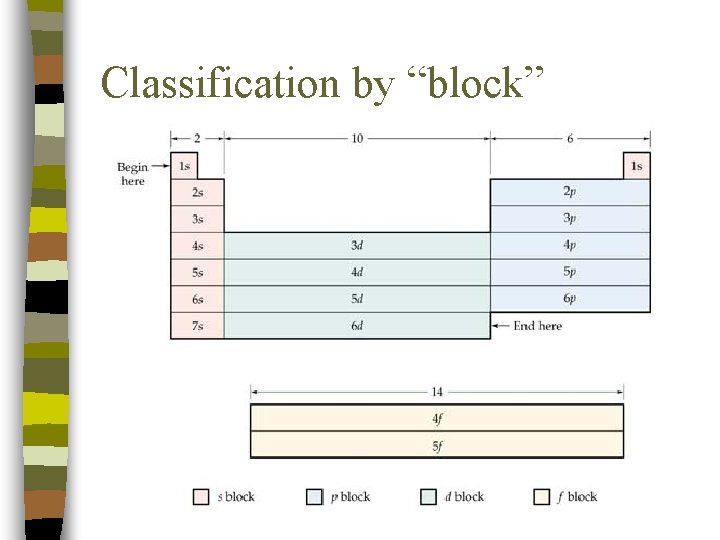
Classification by “block”
Classification by “Block” n n s block: groups IA (electron configuration ends with s 1) & IIA (electron configuration ends with s 2 ) p block: groups IIIA to VIIIA (electron configurations ending with p 1, p 2, p 3, p 4, p 5, & p 6 respectively) – All p block elements have a filled s orbital n d block: transition metals – All d block elements have a filled s orbital BUT. . .
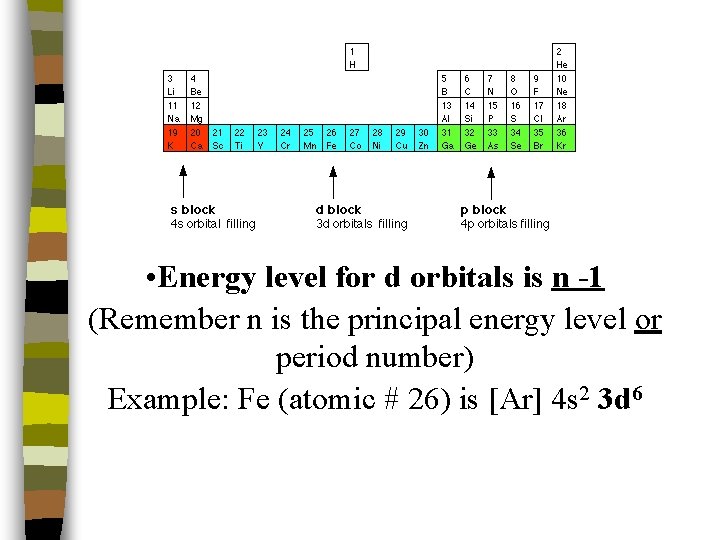
• Energy level for d orbitals is n -1 (Remember n is the principal energy level or period number) Example: Fe (atomic # 26) is [Ar] 4 s 2 3 d 6
Classification by “Block” n f block: Inner transition elements – s sublevel is filled – electron configurations end with n-2 f 1 -14 (Note: the energy level for f orbitals is n-2) n f sublevel does not “fill” with electrons in a predictable way
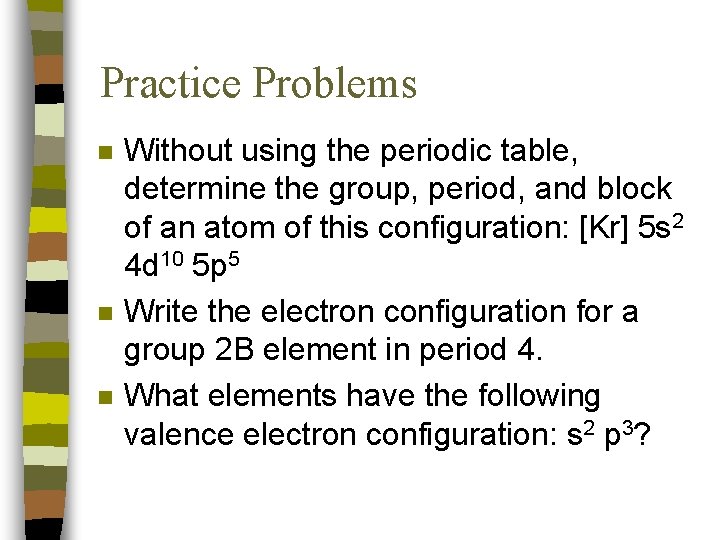
Practice Problems n n n Without using the periodic table, determine the group, period, and block of an atom of this configuration: [Kr] 5 s 2 4 d 10 5 p 5 Write the electron configuration for a group 2 B element in period 4. What elements have the following valence electron configuration: s 2 p 3?
- Slides: 13