Section 5 2 First Law of Thermodynamics Objectives

Section 5. 2 First Law of Thermodynamics

Objectives n n n Explore the 1 st law of thermodynamics Explain why energy cannot be created or destroyed Demonstrate how energy can be transformed and transferred

Key Terms n n n Internal Energy 1 st Law of Thermodynamics Endothermic n n Exothermic State function
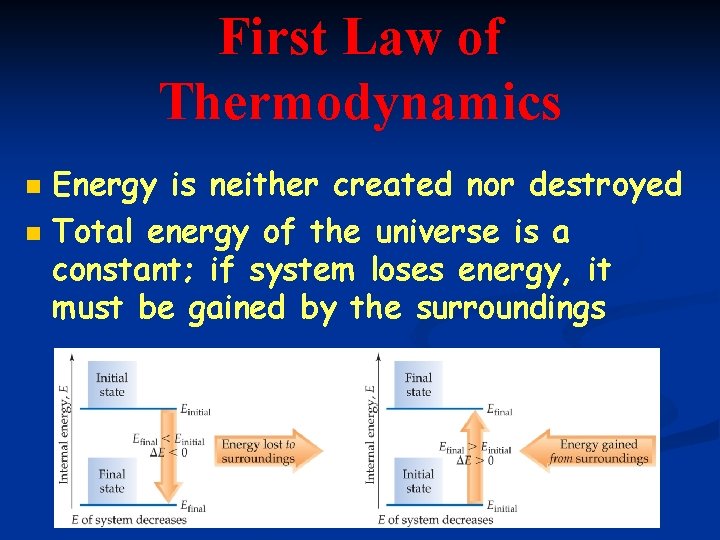
First Law of Thermodynamics n n Energy is neither created nor destroyed Total energy of the universe is a constant; if system loses energy, it must be gained by the surroundings

Internal Energy, E n Sum of all kinetic and potential energies of all components of the system Use Fig. 5. 5
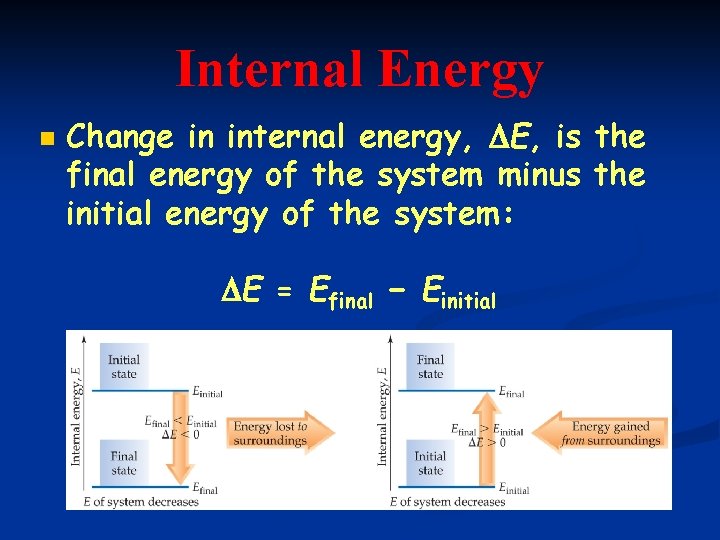
Internal Energy n Change in internal energy, E, is the final energy of the system minus the initial energy of the system: E = Efinal − Einitial Use Fig. 5. 5
E in Chemical Reactions n Einitial state of reactants n Efinal state of products

E > 0 n Efinal > Einitial n System absorbed energy from surroundings n Endergonic

E < 0 n Efinal < Einitial n System released energy to the surroundings n Exergonic
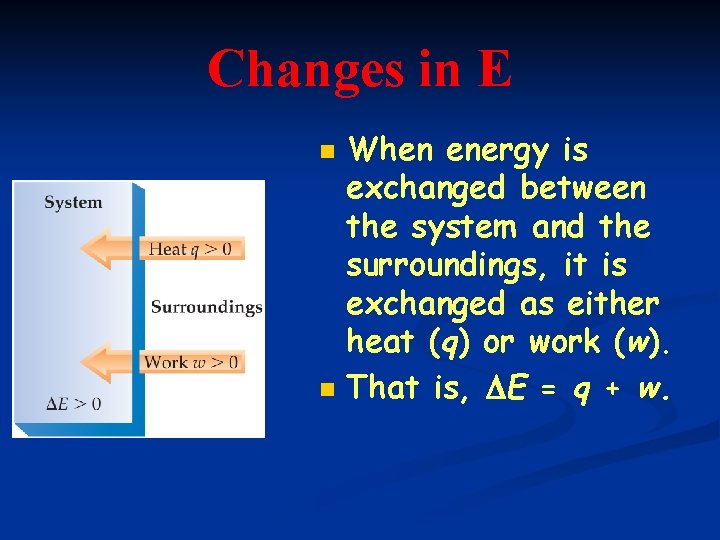
Changes in E n n When energy is exchanged between the system and the surroundings, it is exchanged as either heat (q) or work (w). That is, E = q + w.

E, q, w, and Their Signs
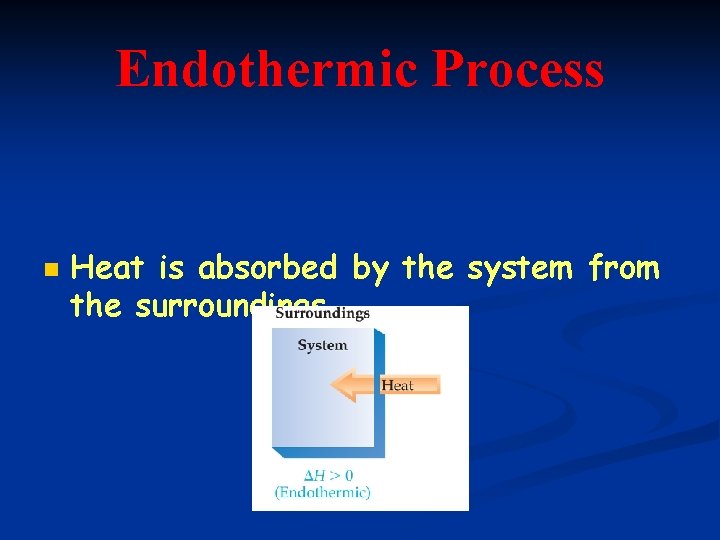
Endothermic Process n Heat is absorbed by the system from the surroundings
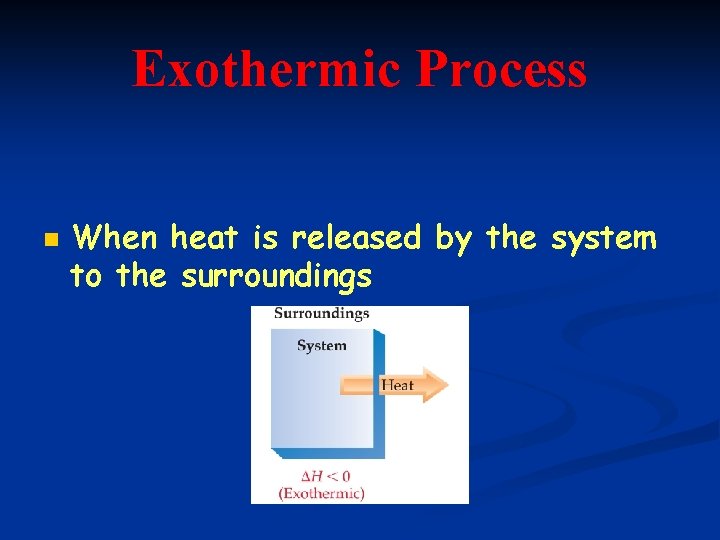
Exothermic Process n When heat is released by the system to the surroundings

State Functions n Property of a system determined by specifying its conditions n Fixed conditions include temperature and pressure
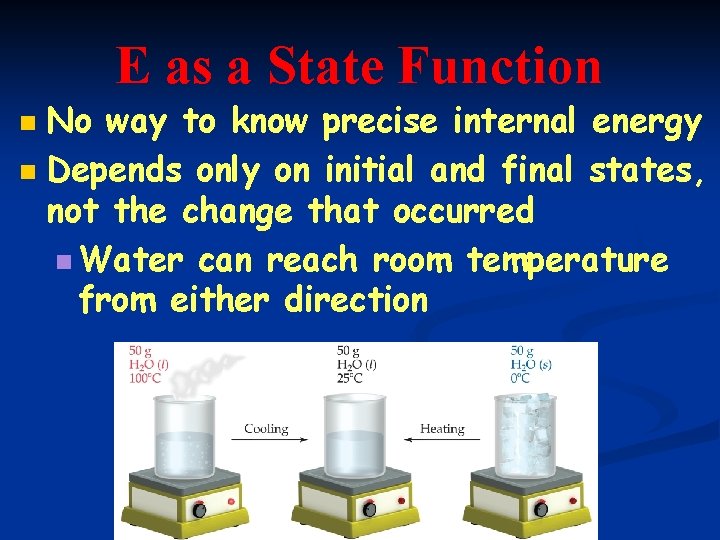
E as a State Function No way to know precise internal energy n Depends only on initial and final states, not the change that occurred n Water can reach room temperature from either direction n

Not State Functions n n q and w E can stay the same, even when q and w are different
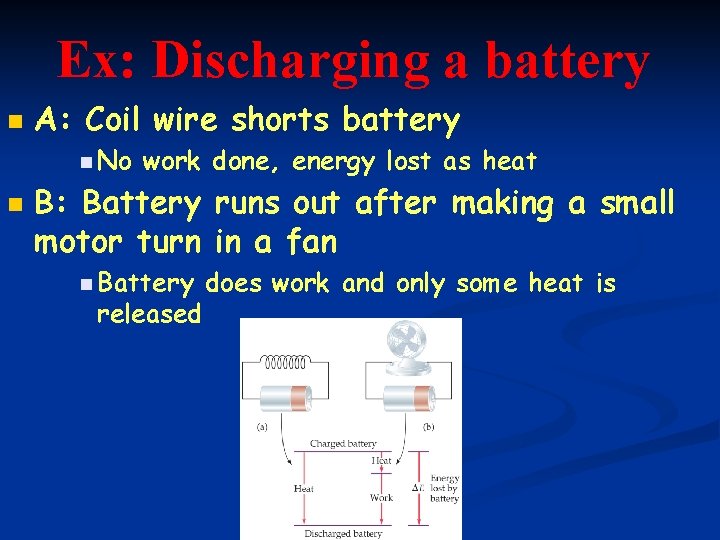
Ex: Discharging a battery n A: Coil wire shorts battery n No n work done, energy lost as heat B: Battery runs out after making a small motor turn in a fan n Battery released does work and only some heat is

A system that absorbs heat from the surroundings is an ____ process. 1. Endothermic 2. Exothermic
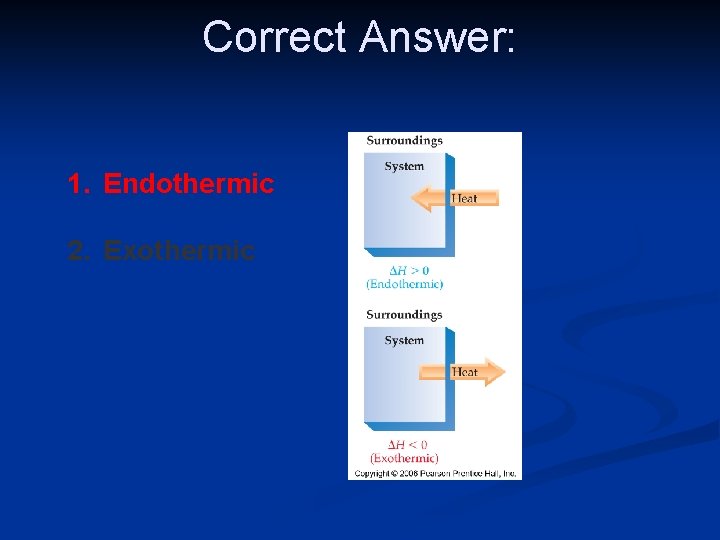
Correct Answer: 1. Endothermic 2. Exothermic

1. 2. 3. 4. It is determined by many factors. It is a numerical value. It is calculated from multiple transactions. It doesn’t depend on how money got into your account or was taken out; it only depends on the net total of all

1. 2. 3. 4. It is determined by many factors. It is a numerical value. It is calculated from multiple transactions. It doesn’t depend on how money got into your account or was taken out; it only depends on the net total of
- Slides: 21