Section 5 1 Light and Quantized Energy Objectives

Section 5. 1 Light and Quantized Energy
Objectives n Compare light n Define the wave and particle models of a quantum of energy and explain how it is related to an energy change of matter n Contrast continuous electromagnetic spectra and atomic emission spectra

Light and Quantized Energy n Problems with Rutherford – Twentieth century scientists found the Rutherford model of the atom incomplete because it did not explain how the electrons were arranged in the atom. – Scientists observed that consecutive elements like chlorine, argon, and potassium have similar number of protons and electrons, but VERY different chemical properties.

Light and Quantized Energy n Wave Nature of Light – Electromagnetic radiation (ER) is a form of energy that exhibits wavelike behavior as it travels through space. – Examples: visible light, microwaves, x-rays, and radio waves.
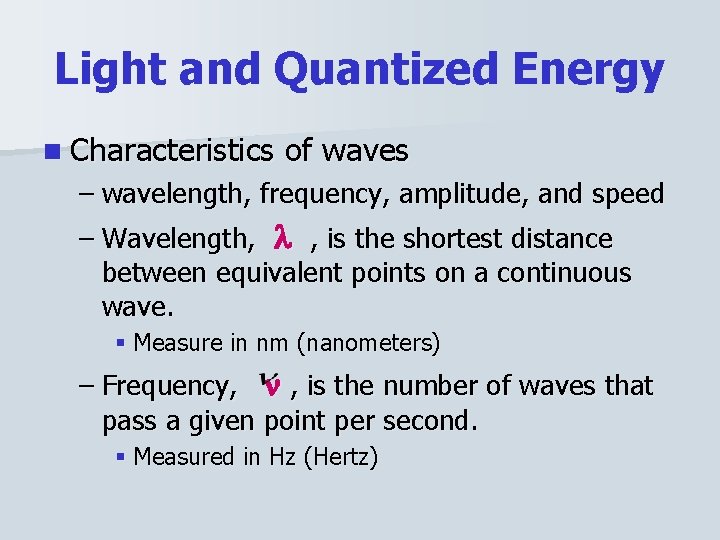
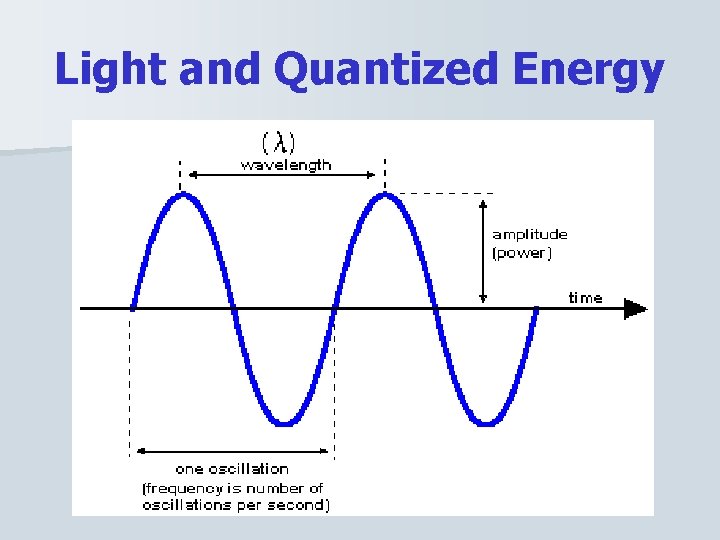
Light and Quantized Energy n Characteristics of waves – wavelength, frequency, amplitude, and speed – Wavelength, , is the shortest distance between equivalent points on a continuous wave. § Measure in nm (nanometers) – Frequency, , is the number of waves that pass a given point per second. § Measured in Hz (Hertz)

Light and Quantized Energy n Characteristics of waves (cont. ) – Amplitude: wave’s height from the origin to a crest, or from the origin to a trough. – The speed of light = c = 3. 0 X 10^8 m/s – Wavelength and Frequency are related to the speed of light by the following equation.
Light and Quantized Energy

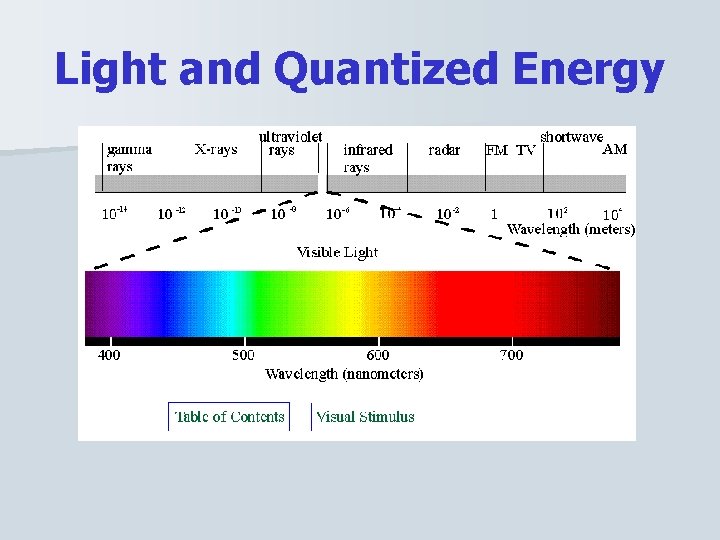
Light and Quantized Energy n Wave Nature of Light – The electromagnetic (EM) spectrum is a continuous spectrum of different types of ER. – We say the EM spectrum is continuous because there is no portion that does not correspond to a unique wavelength and frequency.
Light and Quantized Energy

Light and Quantized Energy n Calculating wavelength/frequency – Example P. 121 – Assignment P. 121 #1 -4
- Slides: 10