Section 4 Thermal Oxidation Jaeger Chapter 3 EE

![The Deal-Grove Model of Oxidation (cont’d) Mass transfer coefficient [cm/sec]. “Fick’s Law of Solid-state The Deal-Grove Model of Oxidation (cont’d) Mass transfer coefficient [cm/sec]. “Fick’s Law of Solid-state](https://slidetodoc.com/presentation_image_h/b2d9df35e537f2371c4591c347b4ab81/image-10.jpg)
![Solution: Oxide Thickness Regimes (Case 1) Large t [ large Xox ] (Case 2) Solution: Oxide Thickness Regimes (Case 1) Large t [ large Xox ] (Case 2)](https://slidetodoc.com/presentation_image_h/b2d9df35e537f2371c4591c347b4ab81/image-20.jpg)
![Local Oxidation of Si [LOCOS] ~100 A Si. O 2 (thermal) - pad oxide Local Oxidation of Si [LOCOS] ~100 A Si. O 2 (thermal) - pad oxide](https://slidetodoc.com/presentation_image_h/b2d9df35e537f2371c4591c347b4ab81/image-37.jpg)
- Slides: 45

Section 4: Thermal Oxidation Jaeger Chapter 3 EE 143 - Ali Javey

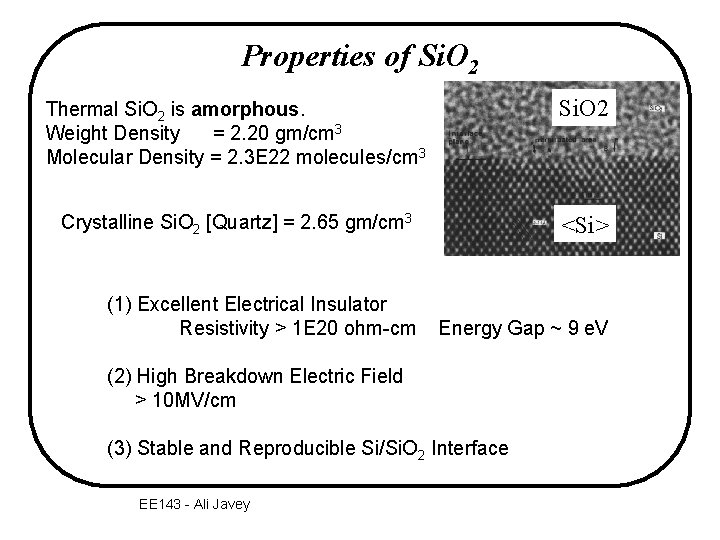
Properties of Si. O 2 Thermal Si. O 2 is amorphous. Weight Density = 2. 20 gm/cm 3 Molecular Density = 2. 3 E 22 molecules/cm 3 Si. O 2 Crystalline Si. O 2 [Quartz] = 2. 65 gm/cm 3 <Si> (1) Excellent Electrical Insulator Resistivity > 1 E 20 ohm-cm Energy Gap ~ 9 e. V (2) High Breakdown Electric Field > 10 MV/cm (3) Stable and Reproducible Si/Si. O 2 Interface EE 143 - Ali Javey

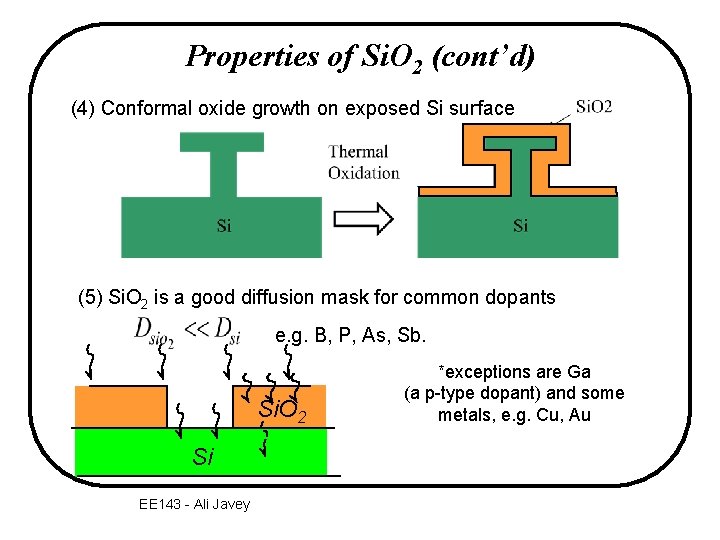
Properties of Si. O 2 (cont’d) (4) Conformal oxide growth on exposed Si surface (5) Si. O 2 is a good diffusion mask for common dopants e. g. B, P, As, Sb. Si. O 2 Si EE 143 - Ali Javey *exceptions are Ga (a p-type dopant) and some metals, e. g. Cu, Au

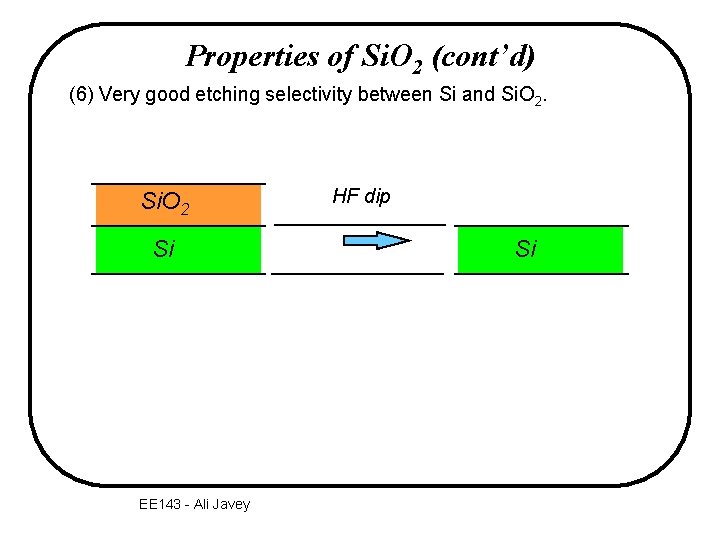
Properties of Si. O 2 (cont’d) (6) Very good etching selectivity between Si and Si. O 2 Si EE 143 - Ali Javey HF dip Si

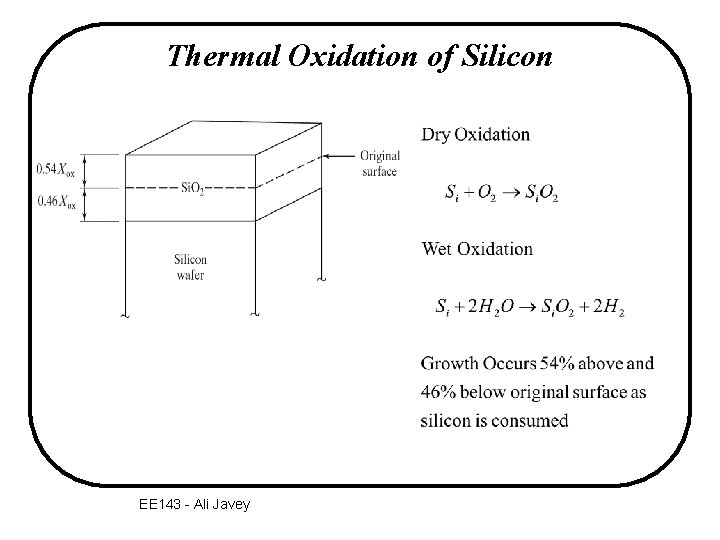
Thermal Oxidation of Silicon EE 143 - Ali Javey

Thermal Oxidation Equipment Horizontal Furnace Vertical Furnace EE 143 - Ali Javey

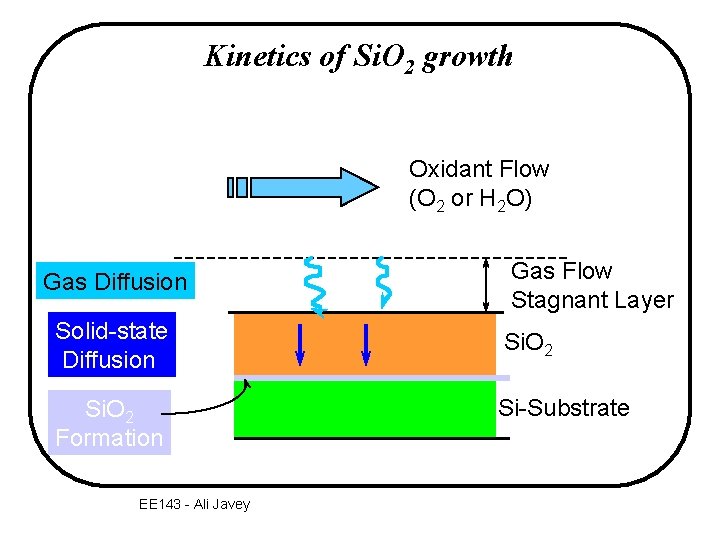
Kinetics of Si. O 2 growth Oxidant Flow (O 2 or H 2 O) Gas Diffusion Gas Flow Stagnant Layer Solid-state Diffusion Si. O 2 Formation Si-Substrate EE 143 - Ali Javey

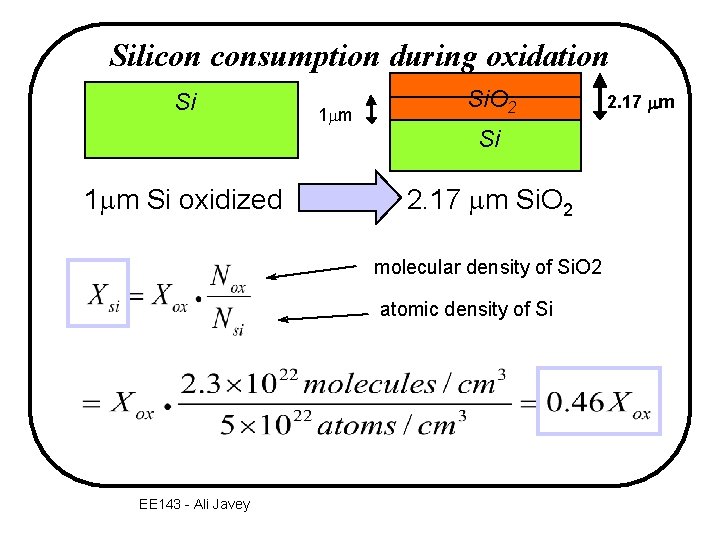
Silicon consumption during oxidation Si 1 mm Si. O 2 Si 1 mm Si oxidized 2. 17 mm Si. O 2 molecular density of Si. O 2 atomic density of Si EE 143 - Ali Javey 2. 17 mm

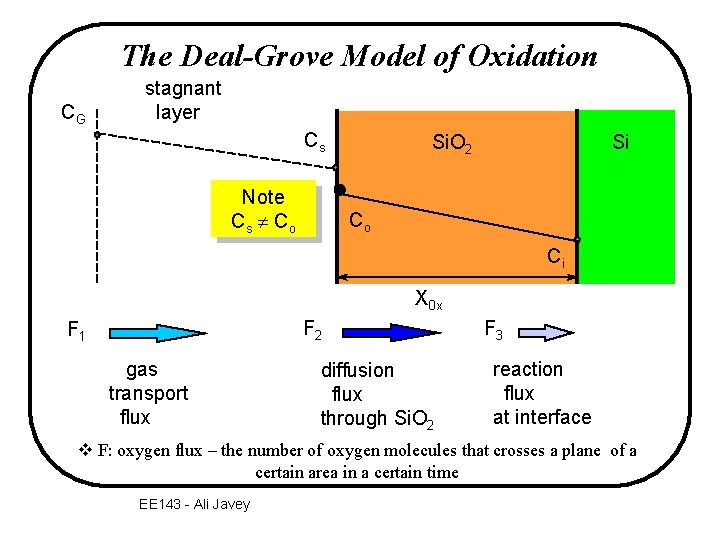
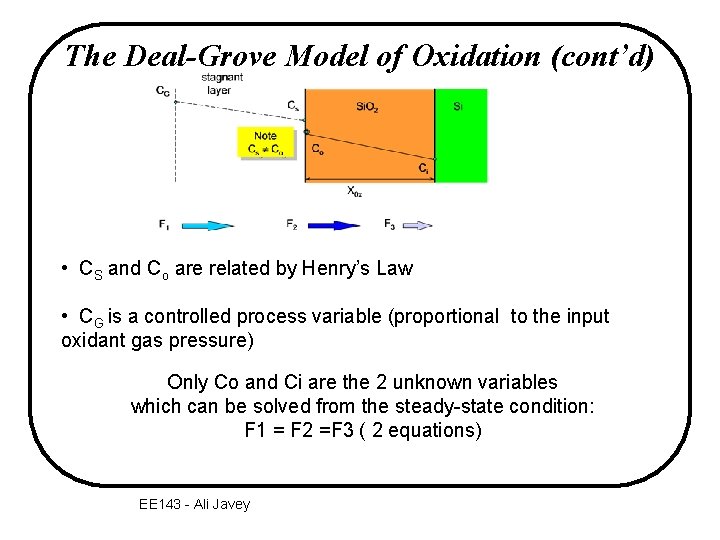
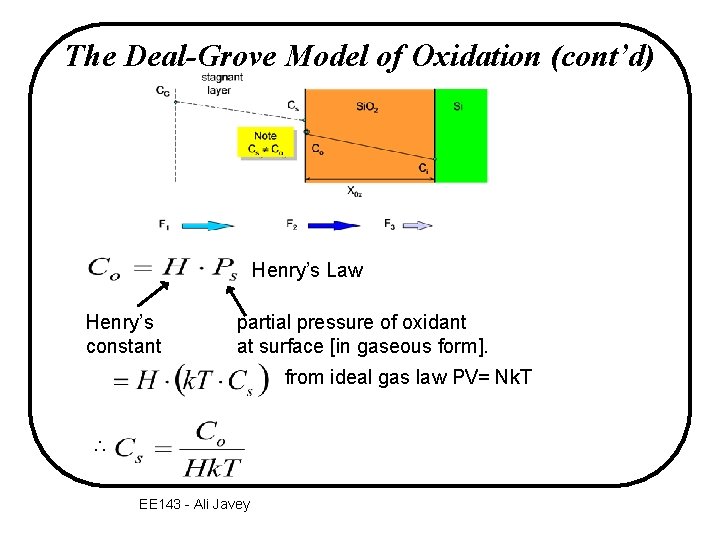
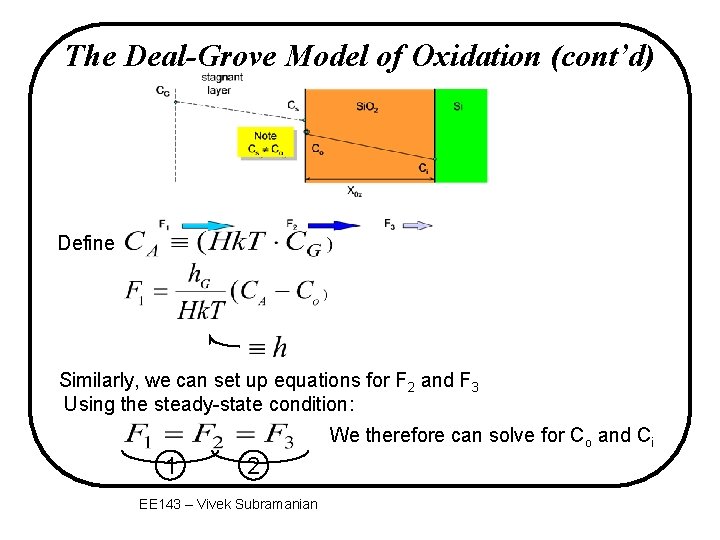
The Deal-Grove Model of Oxidation CG stagnant layer Cs Note Cs C o Si. O 2 Si Co Ci X 0 x F 2 F 1 gas transport flux diffusion flux through Si. O 2 F 3 reaction flux at interface v F: oxygen flux – the number of oxygen molecules that crosses a plane of a certain area in a certain time EE 143 - Ali Javey
![The DealGrove Model of Oxidation contd Mass transfer coefficient cmsec Ficks Law of Solidstate The Deal-Grove Model of Oxidation (cont’d) Mass transfer coefficient [cm/sec]. “Fick’s Law of Solid-state](https://slidetodoc.com/presentation_image_h/b2d9df35e537f2371c4591c347b4ab81/image-10.jpg)
The Deal-Grove Model of Oxidation (cont’d) Mass transfer coefficient [cm/sec]. “Fick’s Law of Solid-state Diffusion” Diffusivity [cm 2/sec] Oxidation reaction rate constant EE 143 - Ali Javey

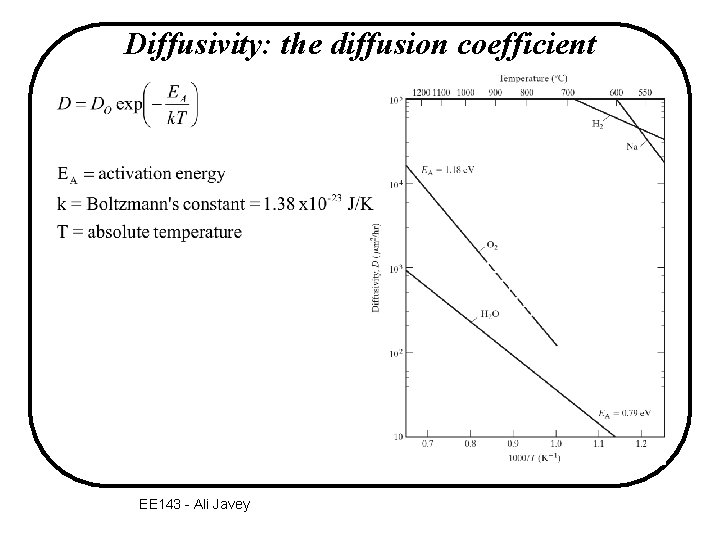
Diffusivity: the diffusion coefficient EE 143 - Ali Javey

The Deal-Grove Model of Oxidation (cont’d) • CS and Co are related by Henry’s Law • CG is a controlled process variable (proportional to the input oxidant gas pressure) Only Co and Ci are the 2 unknown variables which can be solved from the steady-state condition: F 1 = F 2 =F 3 ( 2 equations) EE 143 - Ali Javey

The Deal-Grove Model of Oxidation (cont’d) Henry’s Law Henry’s constant partial pressure of oxidant at surface [in gaseous form]. from ideal gas law PV= Nk. T EE 143 - Ali Javey

The Deal-Grove Model of Oxidation (cont’d) Define Similarly, we can set up equations for F 2 and F 3 Using the steady-state condition: We therefore can solve for Co and Ci 1 2 EE 143 – Vivek Subramanian

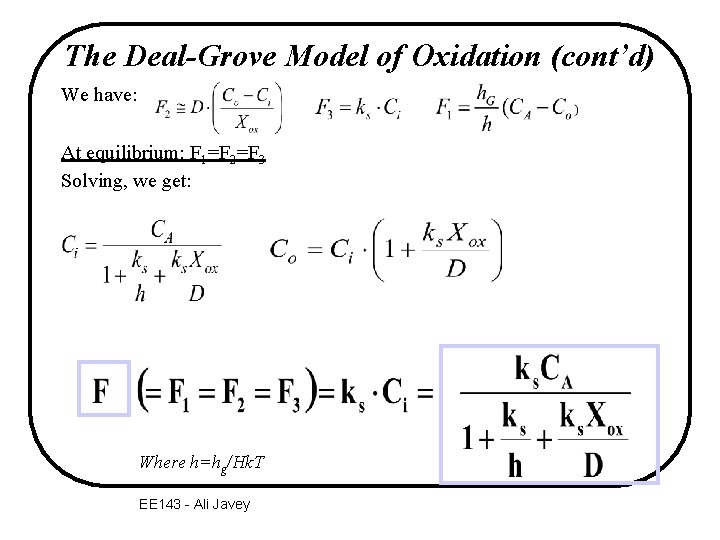
The Deal-Grove Model of Oxidation (cont’d) We have: At equilibrium: F 1=F 2=F 3 Solving, we get: Where h=hg/Hk. T EE 143 - Ali Javey

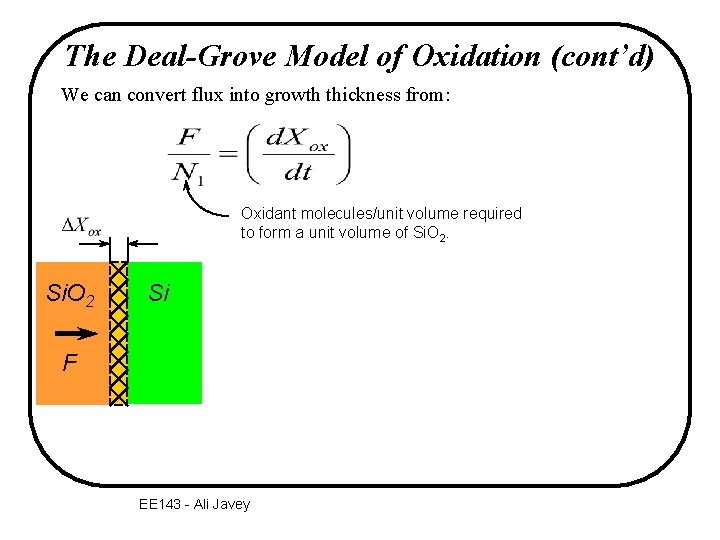
The Deal-Grove Model of Oxidation (cont’d) We can convert flux into growth thickness from: Oxidant molecules/unit volume required to form a unit volume of Si. O 2 Si F EE 143 - Ali Javey

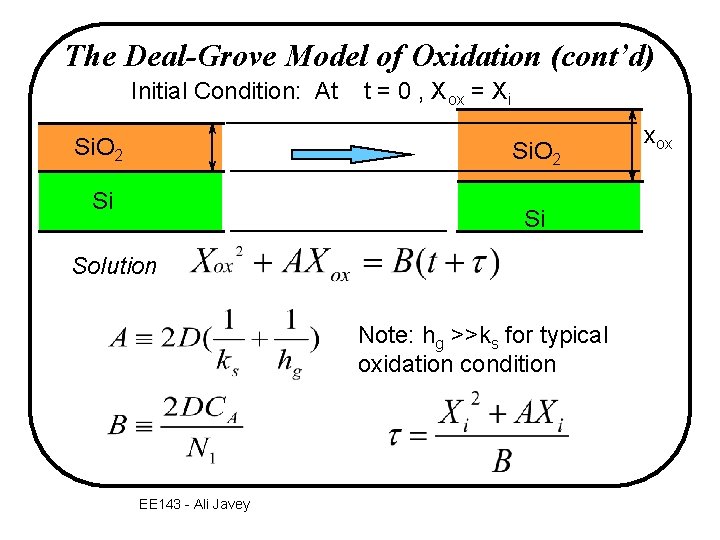
The Deal-Grove Model of Oxidation (cont’d) Initial Condition: At Si. O 2 t = 0 , Xox = Xi Si. O 2 Si Si Solution Note: hg >>ks for typical oxidation condition EE 143 - Ali Javey xox

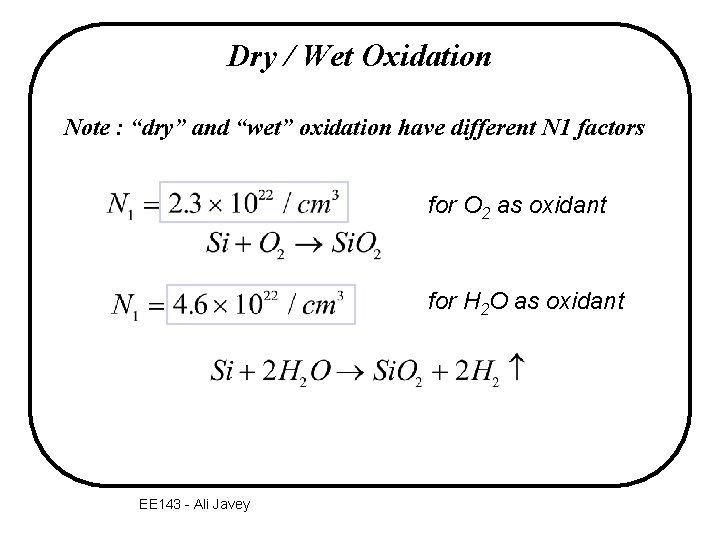
Dry / Wet Oxidation Note : “dry” and “wet” oxidation have different N 1 factors for O 2 as oxidant for H 2 O as oxidant EE 143 - Ali Javey

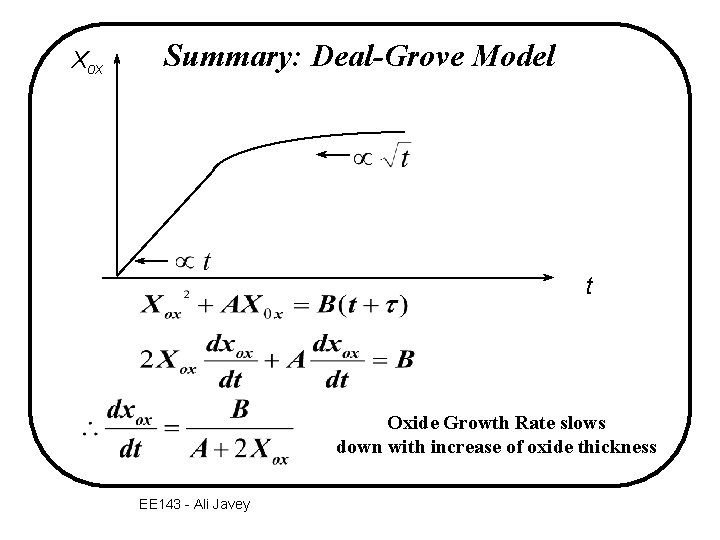
Xox Summary: Deal-Grove Model t Oxide Growth Rate slows down with increase of oxide thickness EE 143 - Ali Javey
![Solution Oxide Thickness Regimes Case 1 Large t large Xox Case 2 Solution: Oxide Thickness Regimes (Case 1) Large t [ large Xox ] (Case 2)](https://slidetodoc.com/presentation_image_h/b2d9df35e537f2371c4591c347b4ab81/image-20.jpg)
Solution: Oxide Thickness Regimes (Case 1) Large t [ large Xox ] (Case 2) Small t [ Small Xox ] EE 143 - Ali Javey

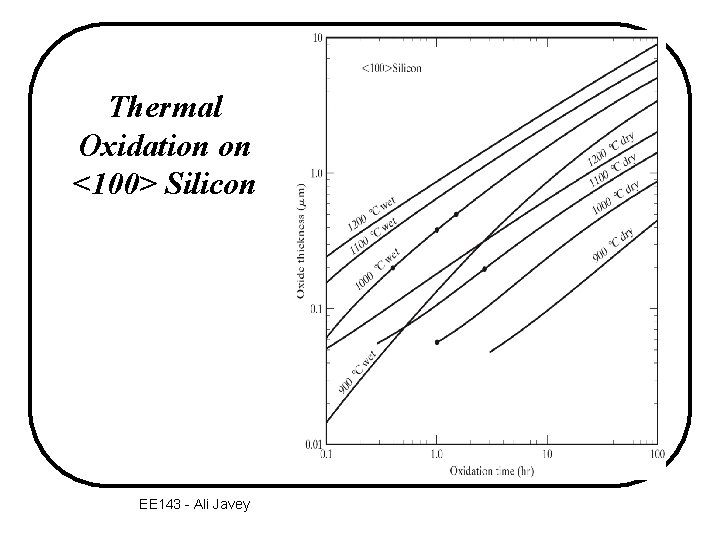
Thermal Oxidation on <100> Silicon EE 143 - Ali Javey

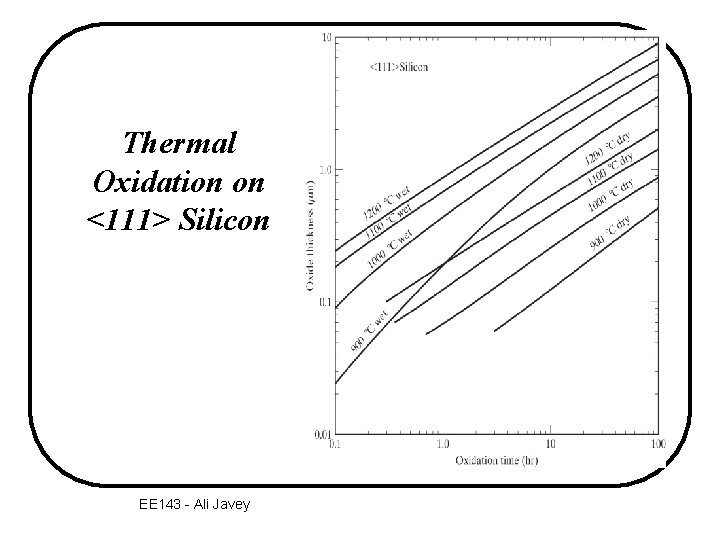
Thermal Oxidation on <111> Silicon EE 143 - Ali Javey

Thermal Oxidation Example A <100> silicon wafer has a 2000 -Å oxide on its surface (a) How long did it take to grow this oxide at 1100 o C in dry oxygen? (b)The wafer is put back in the furnace in wet oxygen at 1000 o C. How long will it take to grow an additional 3000 Å of oxide? EE 143 - Ali Javey

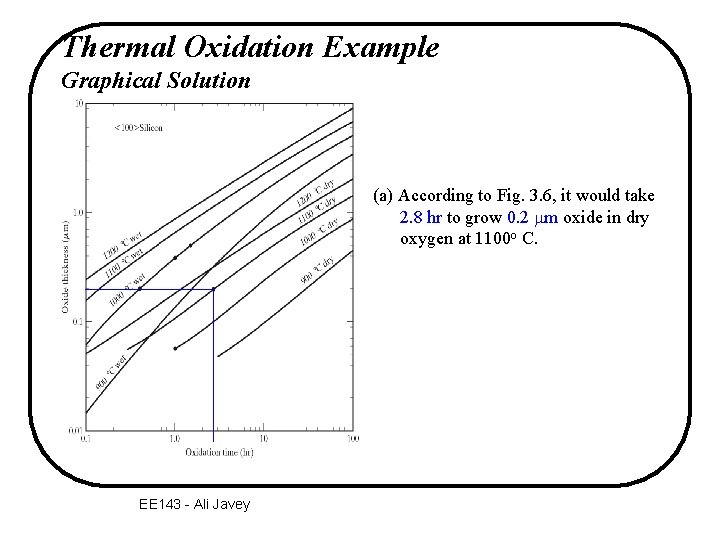
Thermal Oxidation Example Graphical Solution (a) According to Fig. 3. 6, it would take 2. 8 hr to grow 0. 2 mm oxide in dry oxygen at 1100 o C. EE 143 - Ali Javey

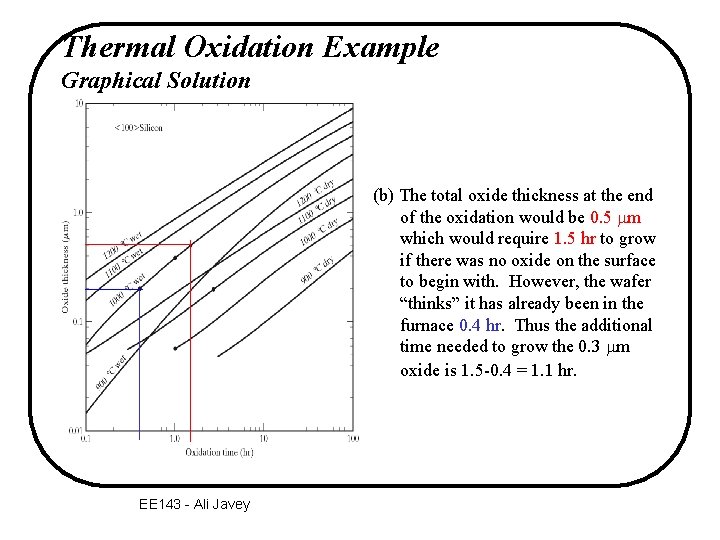
Thermal Oxidation Example Graphical Solution (b) The total oxide thickness at the end of the oxidation would be 0. 5 mm which would require 1. 5 hr to grow if there was no oxide on the surface to begin with. However, the wafer “thinks” it has already been in the furnace 0. 4 hr. Thus the additional time needed to grow the 0. 3 mm oxide is 1. 5 -0. 4 = 1. 1 hr. EE 143 - Ali Javey

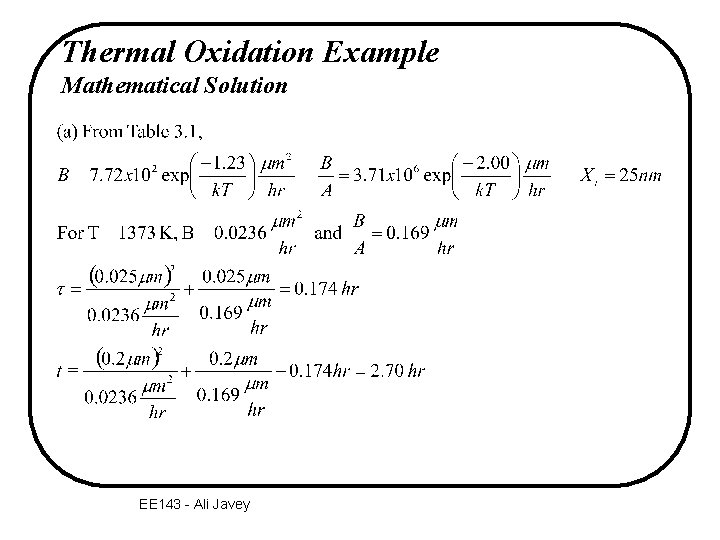
Thermal Oxidation Example Mathematical Solution EE 143 - Ali Javey

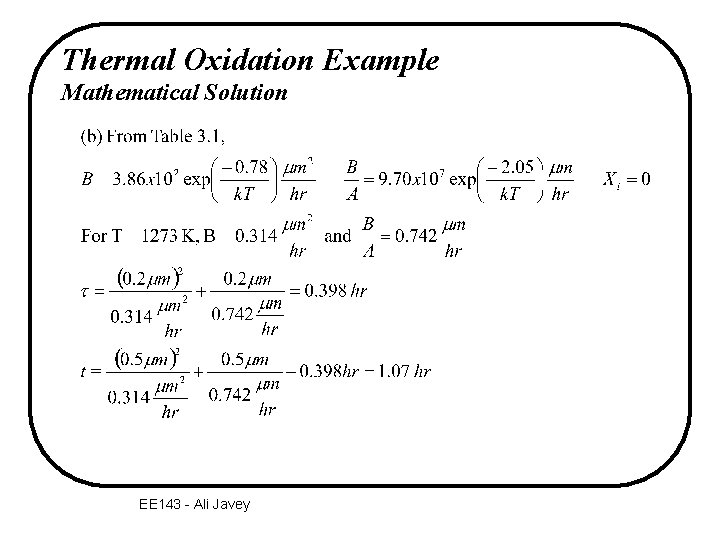
Thermal Oxidation Example Mathematical Solution EE 143 - Ali Javey

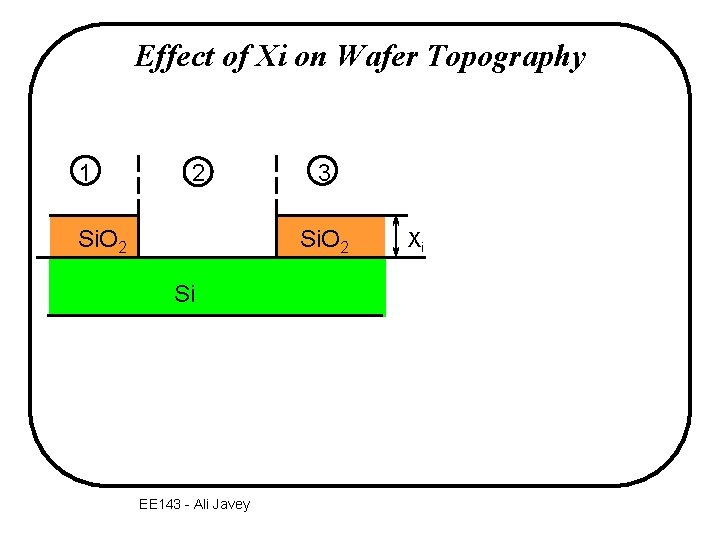
Effect of Xi on Wafer Topography 1 2 Si. O 2 3 Si. O 2 Si EE 143 - Ali Javey Xi

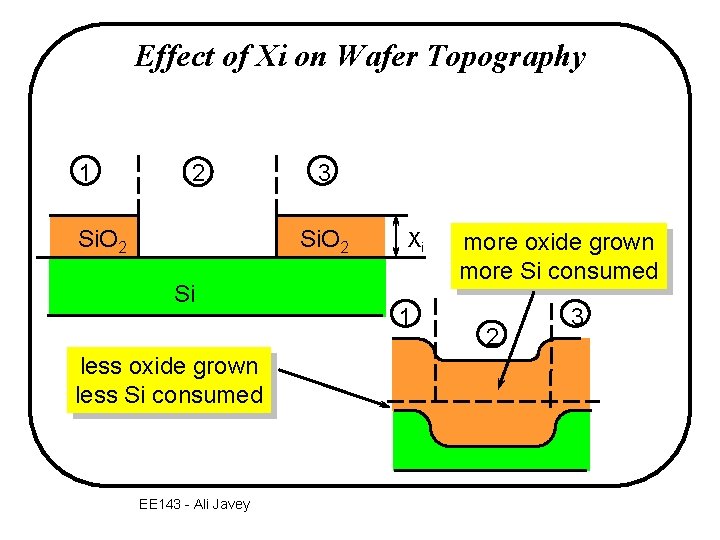
Effect of Xi on Wafer Topography 1 2 Si. O 2 3 Si. O 2 Si less oxide grown less Si consumed EE 143 - Ali Javey Xi 1 more oxide grown more Si consumed 2 3

Factors Influencing Thermal Oxidation – Temperature – Ambient Type (Dry O 2, Steam, HCl) – Ambient Pressure – Substrate Crystallographic Orientation – Substrate Doping EE 143 - Ali Javey

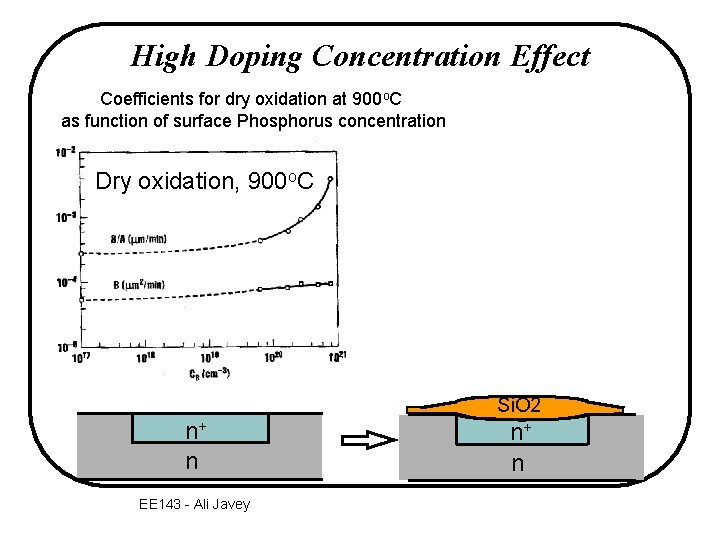
High Doping Concentration Effect Coefficients for dry oxidation at 900 o. C as function of surface Phosphorus concentration Dry oxidation, 900 o. C Si. O 2 n+ n EE 143 - Ali Javey n+ n

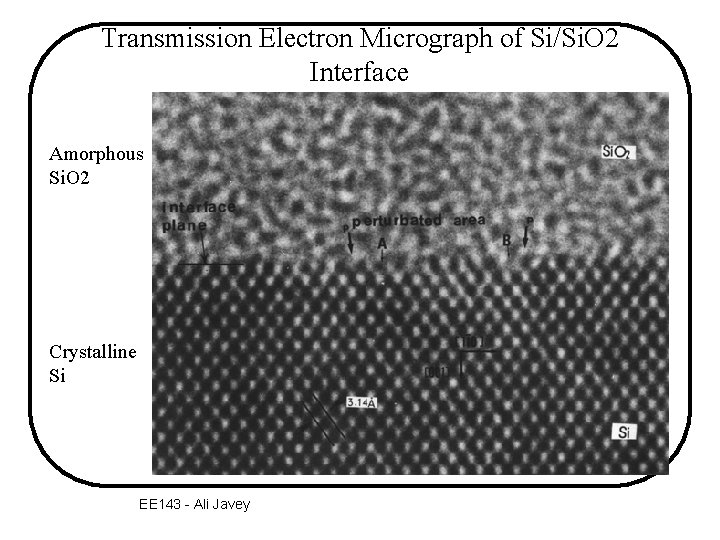
Transmission Electron Micrograph of Si/Si. O 2 Interface Amorphous Si. O 2 Crystalline Si EE 143 - Ali Javey

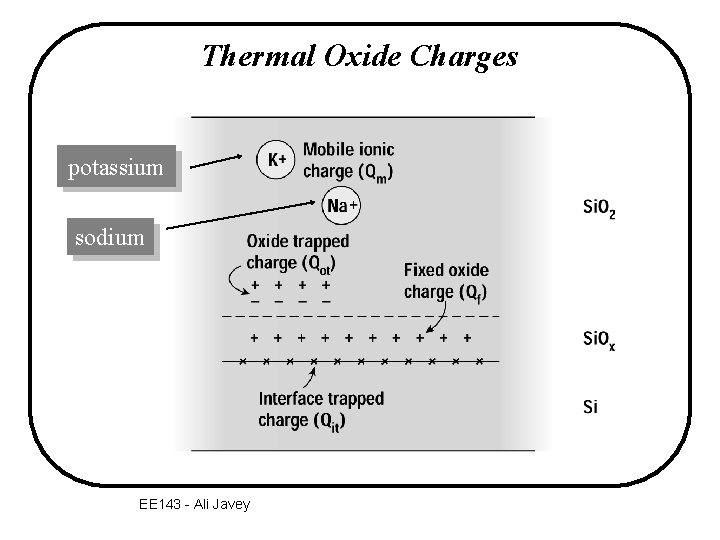
Thermal Oxide Charges potassium sodium EE 143 - Ali Javey

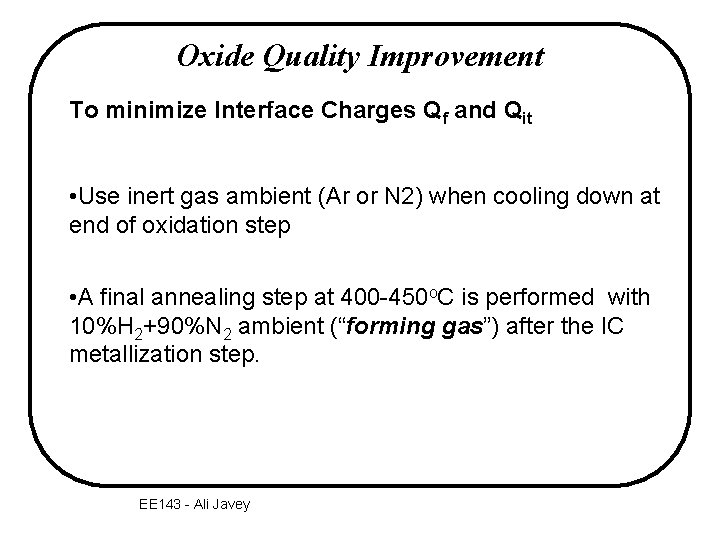
Oxide Quality Improvement To minimize Interface Charges Qf and Qit • Use inert gas ambient (Ar or N 2) when cooling down at end of oxidation step • A final annealing step at 400 -450 o. C is performed with 10%H 2+90%N 2 ambient (“forming gas”) after the IC metallization step. EE 143 - Ali Javey

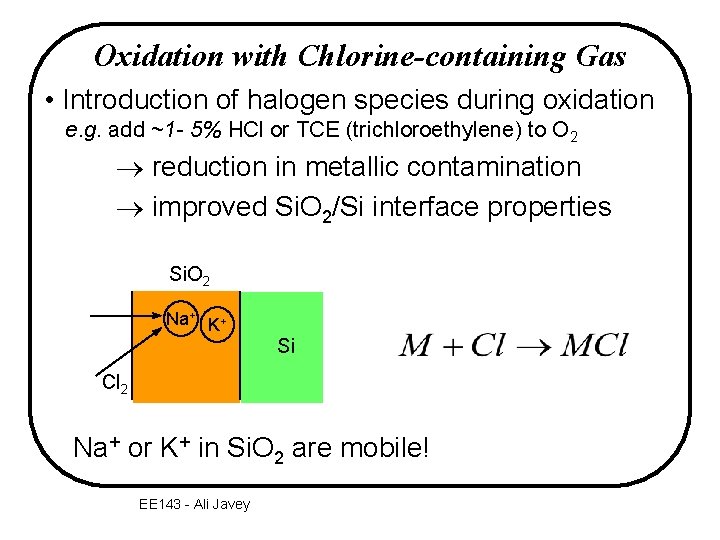
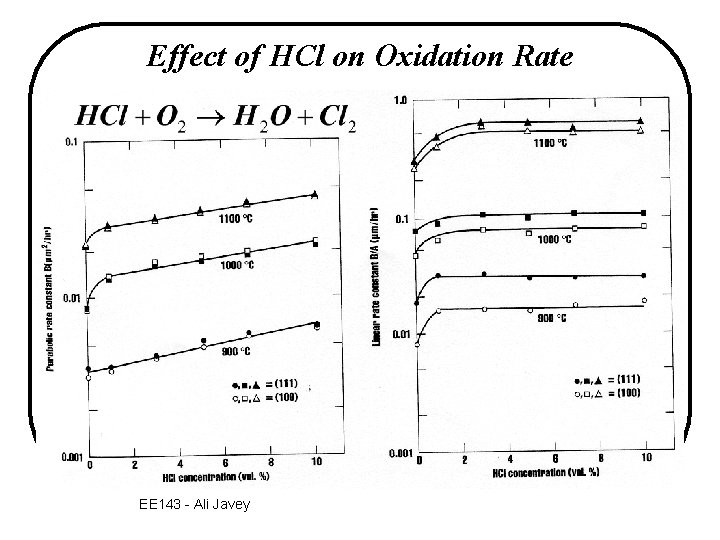
Oxidation with Chlorine-containing Gas • Introduction of halogen species during oxidation e. g. add ~1 - 5% HCl or TCE (trichloroethylene) to O 2 ® reduction in metallic contamination ® improved Si. O 2/Si interface properties Si. O 2 Na+ K+ Si Cl 2 Na+ or K+ in Si. O 2 are mobile! EE 143 - Ali Javey

Effect of HCl on Oxidation Rate EE 143 - Ali Javey
![Local Oxidation of Si LOCOS 100 A Si O 2 thermal pad oxide Local Oxidation of Si [LOCOS] ~100 A Si. O 2 (thermal) - pad oxide](https://slidetodoc.com/presentation_image_h/b2d9df35e537f2371c4591c347b4ab81/image-37.jpg)
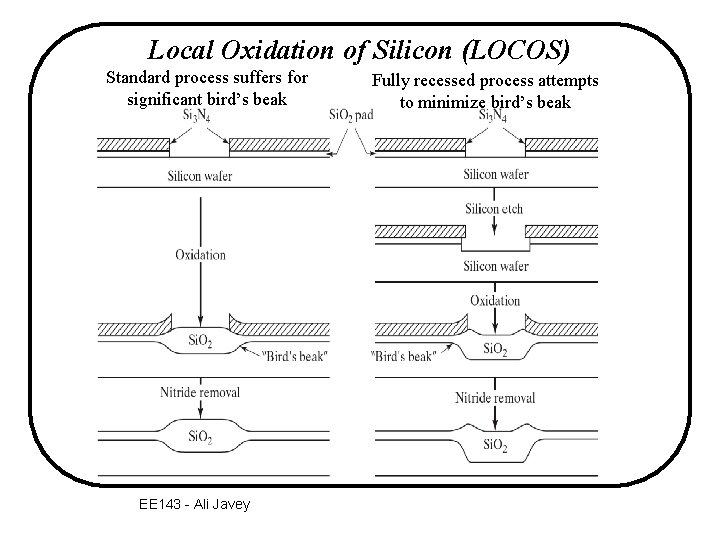
Local Oxidation of Si [LOCOS] ~100 A Si. O 2 (thermal) - pad oxide to release mechanical stress between nitride and Si. Oxidation Nitride Etch EE 143 - Ali Javey

Local Oxidation of Silicon (LOCOS) Standard process suffers for significant bird’s beak EE 143 - Ali Javey Fully recessed process attempts to minimize bird’s beak

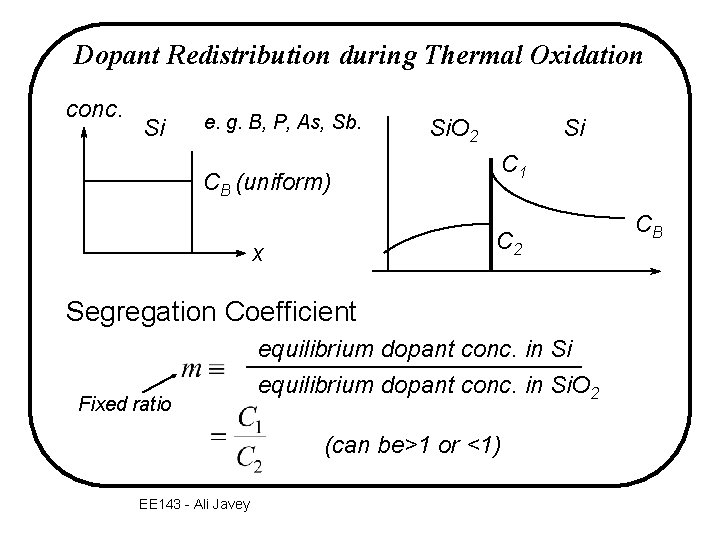
Dopant Redistribution during Thermal Oxidation conc. Si e. g. B, P, As, Sb. CB (uniform) Si Si. O 2 C 1 C 2 x Segregation Coefficient Fixed ratio equilibrium dopant conc. in Si. O 2 (can be>1 or <1) EE 143 - Ali Javey CB

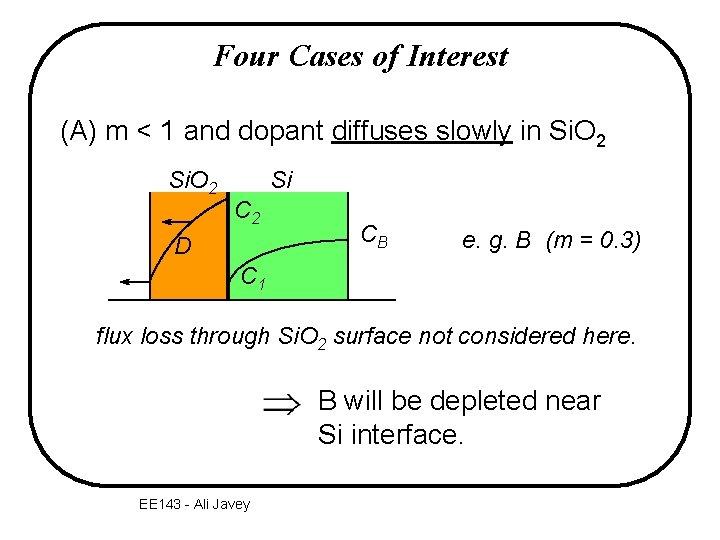
Four Cases of Interest (A) m < 1 and dopant diffuses slowly in Si. O 2 Si C 2 D CB e. g. B (m = 0. 3) C 1 flux loss through Si. O 2 surface not considered here. B will be depleted near Si interface. EE 143 - Ali Javey

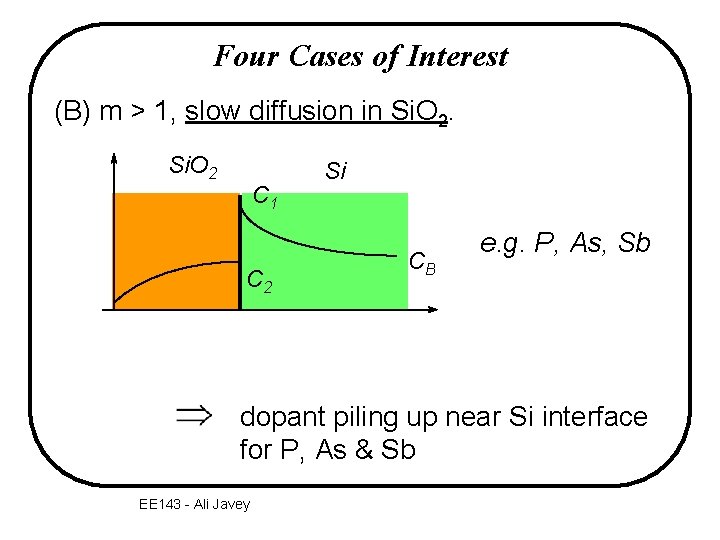
Four Cases of Interest (B) m > 1, slow diffusion in Si. O 2 C 1 C 2 Si CB e. g. P, As, Sb dopant piling up near Si interface for P, As & Sb EE 143 - Ali Javey

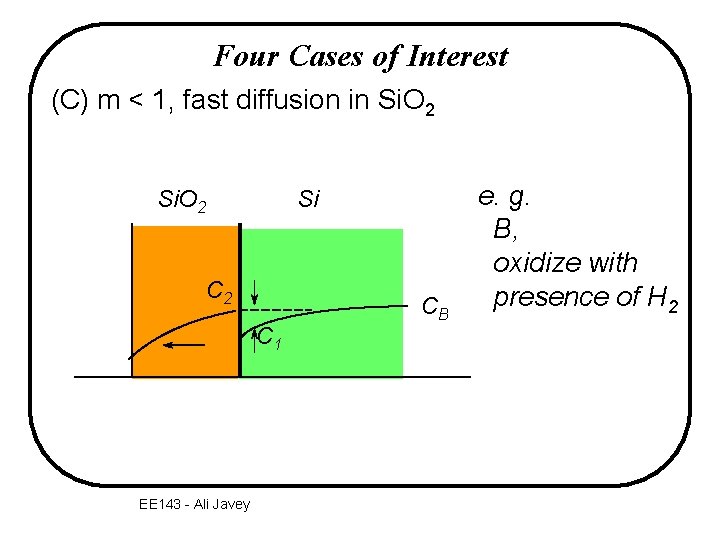
Four Cases of Interest (C) m < 1, fast diffusion in Si. O 2 Si C 2 C 1 EE 143 - Ali Javey CB e. g. B, oxidize with presence of H 2

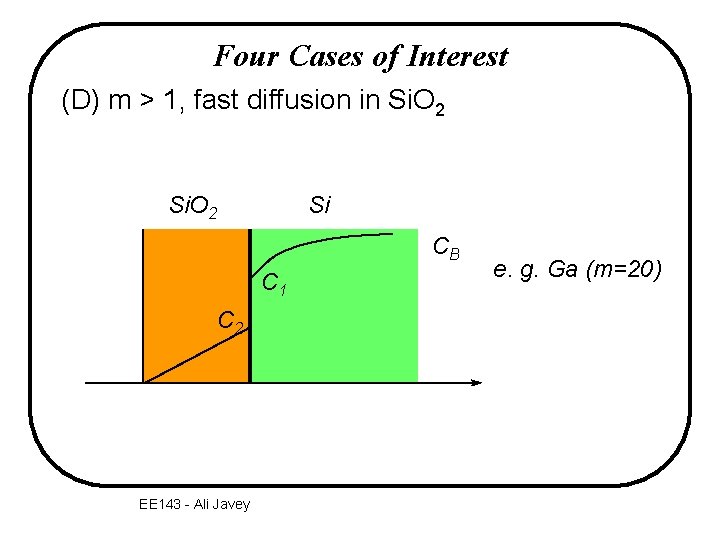
Four Cases of Interest (D) m > 1, fast diffusion in Si. O 2 Si CB C 1 C 2 EE 143 - Ali Javey e. g. Ga (m=20)

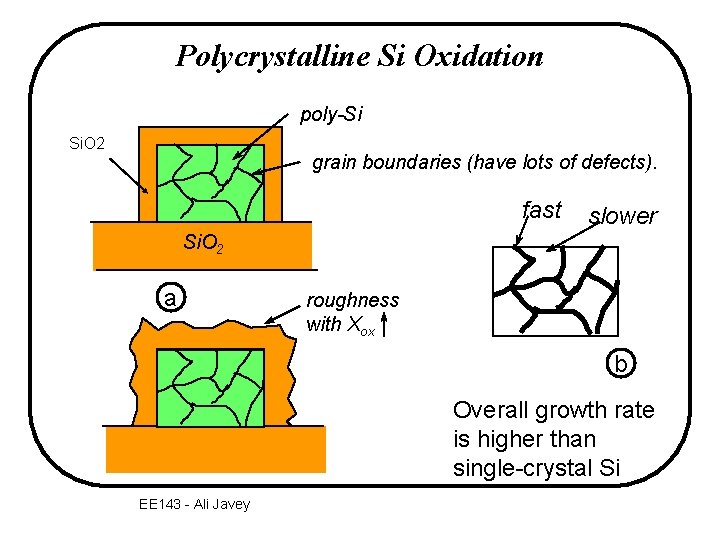
Polycrystalline Si Oxidation poly-Si Si. O 2 grain boundaries (have lots of defects). fast slower Si. O 2 a roughness with Xox b Overall growth rate is higher than single-crystal Si EE 143 - Ali Javey

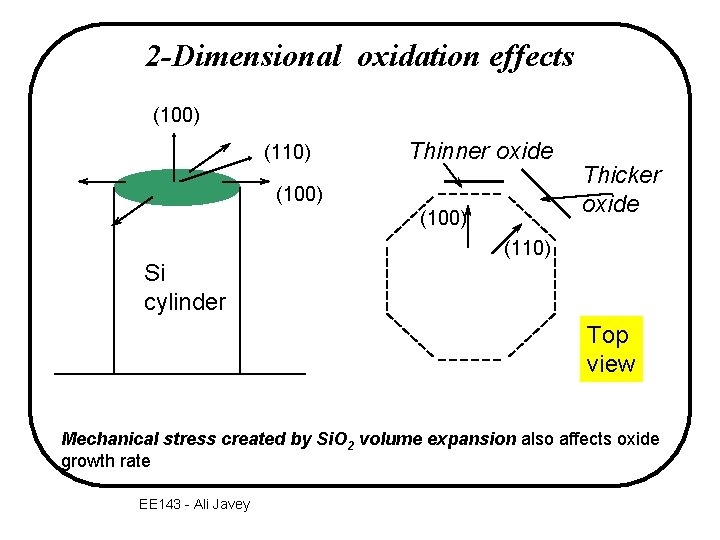
2 -Dimensional oxidation effects (100) (110) Thinner oxide (100) Thicker oxide (110) Si cylinder Top view Mechanical stress created by Si. O 2 volume expansion also affects oxide growth rate EE 143 - Ali Javey