Section 4 Calculating Enthalpy Change The enthalpy change

- Slides: 14

Section 4: Calculating Enthalpy Change The enthalpy change for a reaction can be calculated using Hess’s law. K What I Know W What I Want to Find Out L What I Learned

• 11(C) Use thermochemical equations to calculate energy changes that occur in chemical reactions and classify reactions as exothermic or endothermic. Copyright © Mc. Graw-Hill Education Calculating Enthalpy Change

Essential Questions • How is Hess’s law applied to calculate the enthalpy change for a reaction? • What is the basis for the table of standard enthalpies of formation? • What is the enthalpy change for a reaction using standard enthalpies of formation data? Copyright © Mc. Graw-Hill Education Calculating Enthalpy Change

Vocabulary Review New • allotrope • Hess’s law • standard enthalpy (heat) of formation Copyright © Mc. Graw-Hill Education Calculating Enthalpy Change

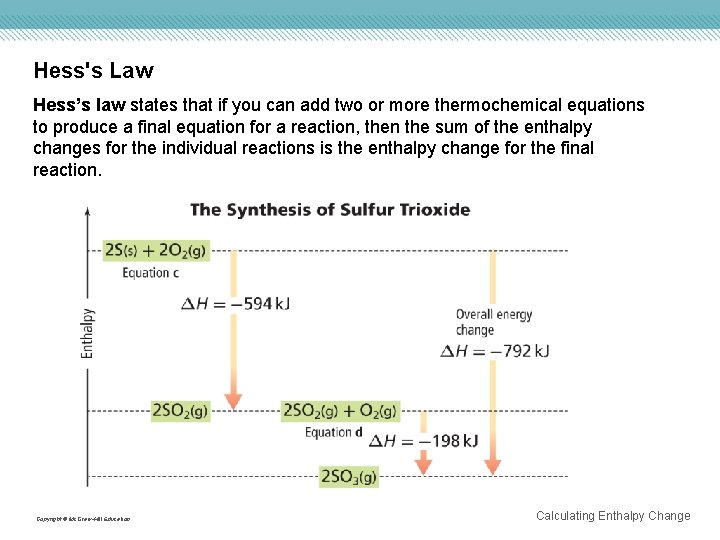
Hess's Law Hess’s law states that if you can add two or more thermochemical equations to produce a final equation for a reaction, then the sum of the enthalpy changes for the individual reactions is the enthalpy change for the final reaction. Copyright © Mc. Graw-Hill Education Calculating Enthalpy Change

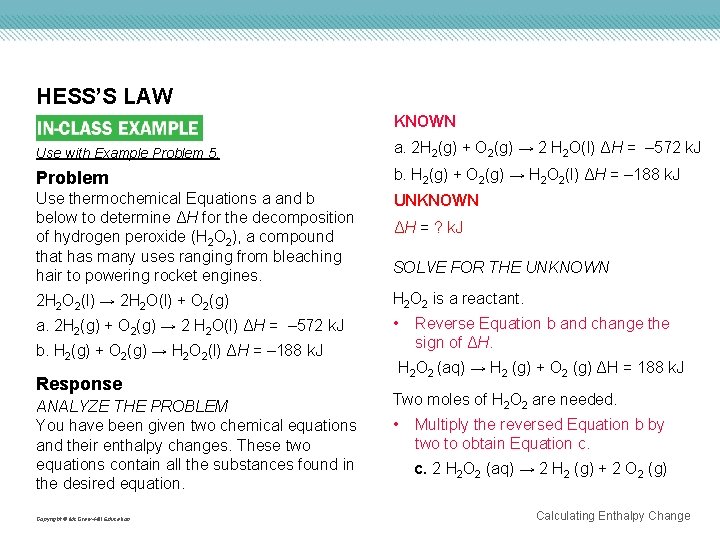
HESS’S LAW KNOWN Use with Example Problem 5. a. 2 H 2(g) + O 2(g) → 2 H 2 O(l) ΔH = – 572 k. J Problem b. H 2(g) + O 2(g) → H 2 O 2(l) ΔH = – 188 k. J Use thermochemical Equations a and b below to determine ΔH for the decomposition of hydrogen peroxide (H 2 O 2), a compound that has many uses ranging from bleaching hair to powering rocket engines. UNKNOWN ΔH = ? k. J SOLVE FOR THE UNKNOWN 2 H 2 O 2(l) → 2 H 2 O(l) + O 2(g) H 2 O 2 is a reactant. a. 2 H 2(g) + O 2(g) → 2 H 2 O(l) ΔH = – 572 k. J • b. H 2(g) + O 2(g) → H 2 O 2(l) ΔH = – 188 k. J Response ANALYZE THE PROBLEM You have been given two chemical equations and their enthalpy changes. These two equations contain all the substances found in the desired equation. Copyright © Mc. Graw-Hill Education Reverse Equation b and change the sign of ΔH. H 2 O 2 (aq) → H 2 (g) + O 2 (g) ΔH = 188 k. J Two moles of H 2 O 2 are needed. • Multiply the reversed Equation b by two to obtain Equation c. c. 2 H 2 O 2 (aq) → 2 H 2 (g) + 2 O 2 (g) Calculating Enthalpy Change

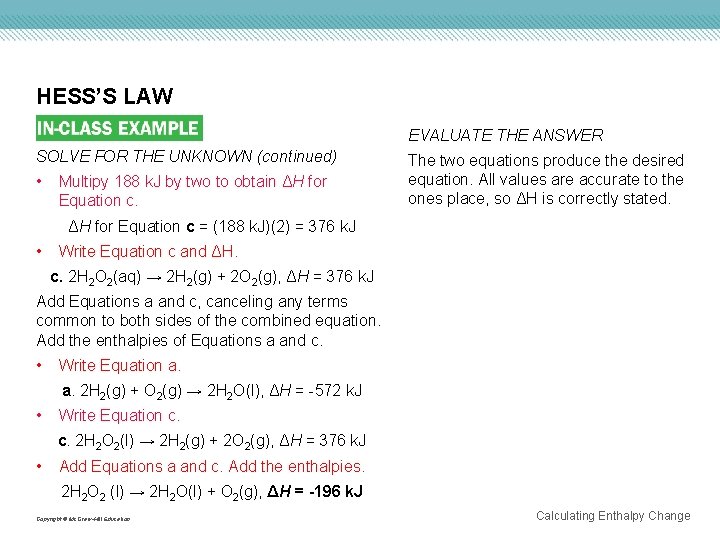
HESS’S LAW EVALUATE THE ANSWER SOLVE FOR THE UNKNOWN (continued) • Multipy 188 k. J by two to obtain ΔH for Equation c. The two equations produce the desired equation. All values are accurate to the ones place, so ΔH is correctly stated. ΔH for Equation c = (188 k. J)(2) = 376 k. J • Write Equation c and ΔH. c. 2 H 2 O 2(aq) → 2 H 2(g) + 2 O 2(g), ΔH = 376 k. J Add Equations a and c, canceling any terms common to both sides of the combined equation. Add the enthalpies of Equations a and c. • Write Equation a. a. 2 H 2(g) + O 2(g) → 2 H 2 O(l), ΔH = -572 k. J • Write Equation c. c. 2 H 2 O 2(l) → 2 H 2(g) + 2 O 2(g), ΔH = 376 k. J • Add Equations a and c. Add the enthalpies. 2 H 2 O 2 (l) → 2 H 2 O(l) + O 2(g), ΔH = -196 k. J Copyright © Mc. Graw-Hill Education Calculating Enthalpy Change

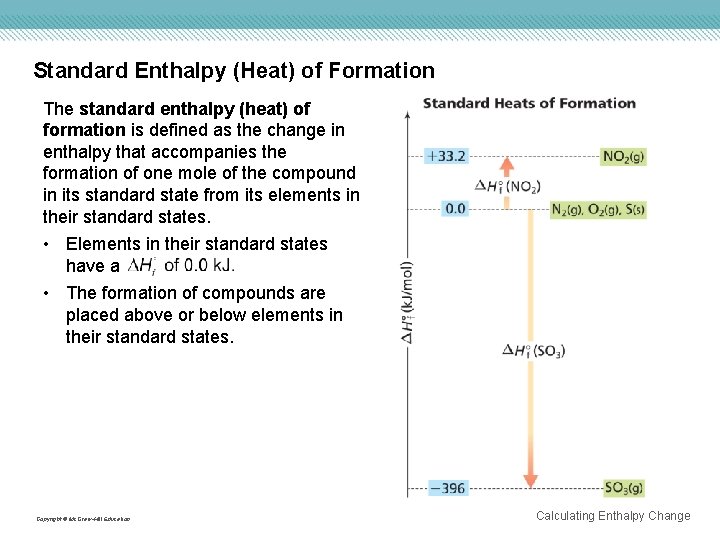
Standard Enthalpy (Heat) of Formation The standard enthalpy (heat) of formation is defined as the change in enthalpy that accompanies the formation of one mole of the compound in its standard state from its elements in their standard states. • Elements in their standard states have a • The formation of compounds are placed above or below elements in their standard states. Copyright © Mc. Graw-Hill Education Calculating Enthalpy Change

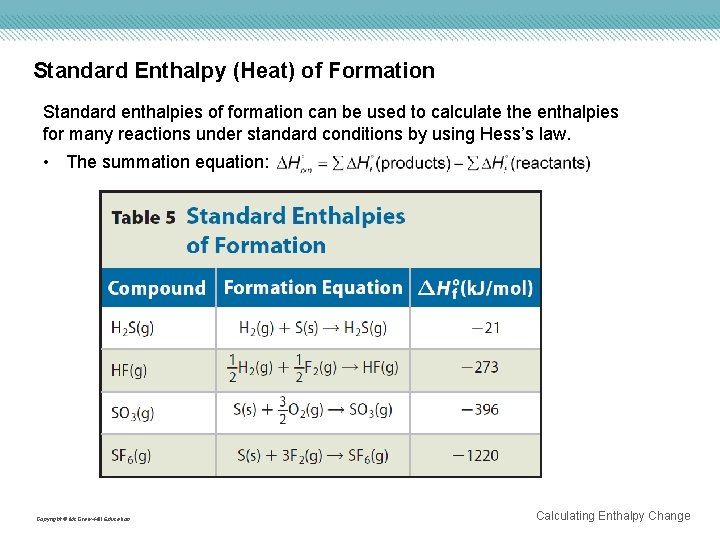
Standard Enthalpy (Heat) of Formation Standard enthalpies of formation can be used to calculate the enthalpies for many reactions under standard conditions by using Hess’s law. • The summation equation: Copyright © Mc. Graw-Hill Education Calculating Enthalpy Change

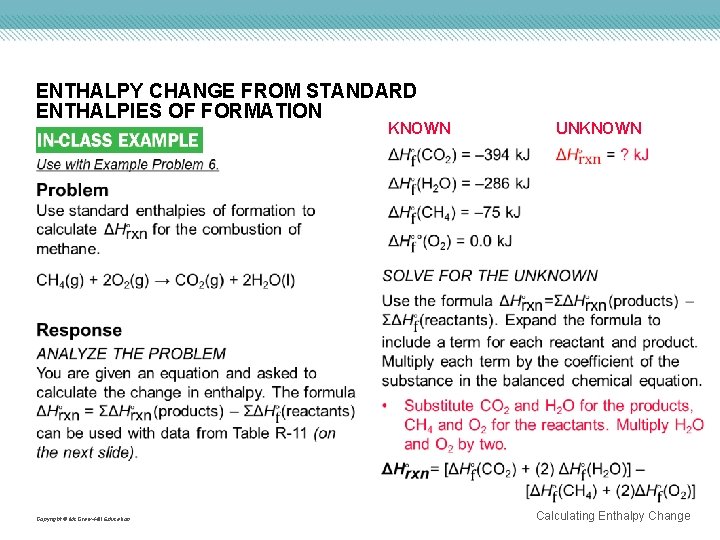
ENTHALPY CHANGE FROM STANDARD ENTHALPIES OF FORMATION KNOWN Copyright © Mc. Graw-Hill Education UNKNOWN Calculating Enthalpy Change

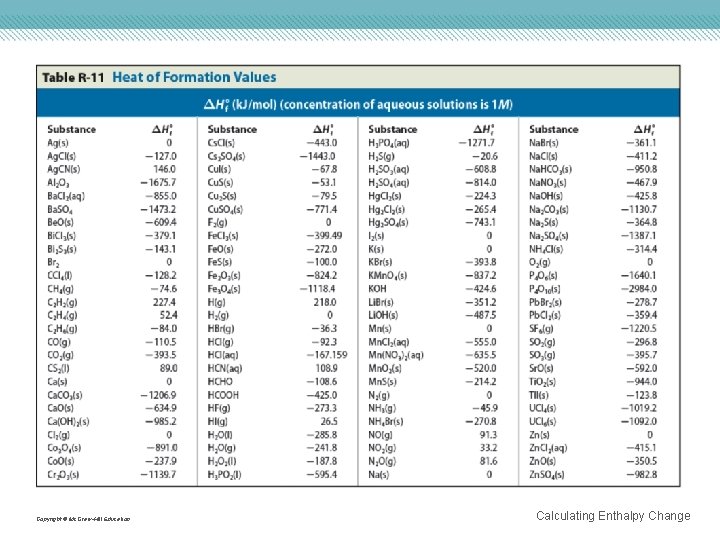
Copyright © Mc. Graw-Hill Education Calculating Enthalpy Change

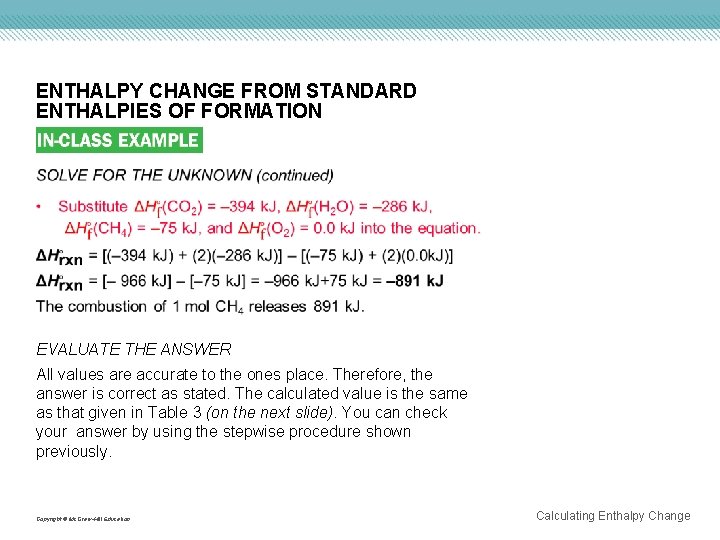
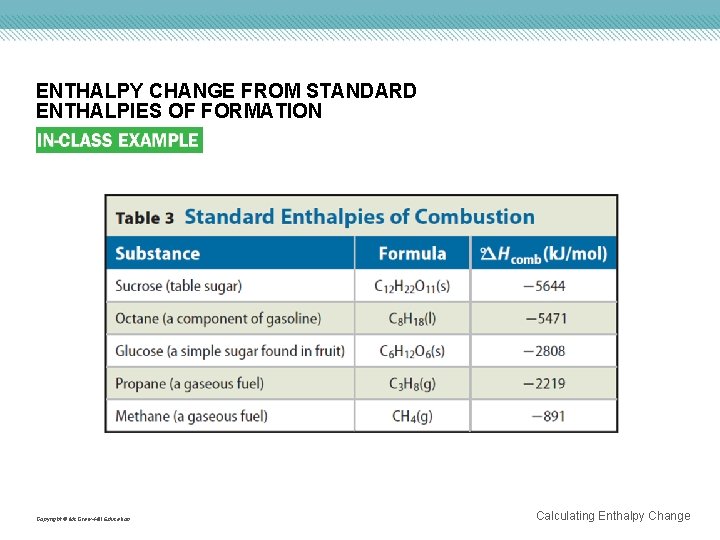
ENTHALPY CHANGE FROM STANDARD ENTHALPIES OF FORMATION EVALUATE THE ANSWER All values are accurate to the ones place. Therefore, the answer is correct as stated. The calculated value is the same as that given in Table 3 (on the next slide). You can check your answer by using the stepwise procedure shown previously. Copyright © Mc. Graw-Hill Education Calculating Enthalpy Change

ENTHALPY CHANGE FROM STANDARD ENTHALPIES OF FORMATION Copyright © Mc. Graw-Hill Education Calculating Enthalpy Change

Review Essential Questions • How is Hess’s law applied to calculate the enthalpy change for a reaction? • What is the basis for the table of standard enthalpies of formation? • What is the enthalpy change for a reaction using standard enthalpies of formation data? Vocabulary • Hess’s law Copyright © Mc. Graw-Hill Education • standard enthalpy (heat) of formation Calculating Enthalpy Change