Section 4 9 OxidationReduction Reactions Redox Reactions Reactions










- Slides: 15

Section 4. 9 Oxidation-Reduction Reactions Redox Reactions § Reactions in which one or more electrons are transferred. Copyright © Cengage Learning. All rights reserved 1

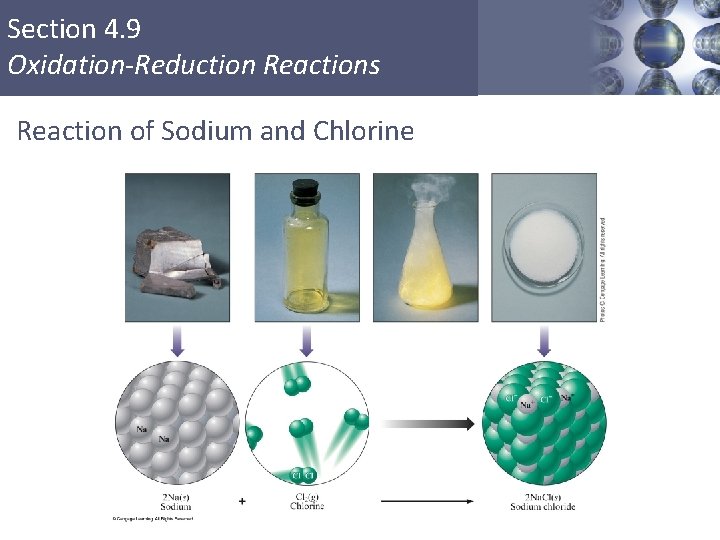
Section 4. 9 Oxidation-Reduction Reactions Reaction of Sodium and Chlorine Copyright © Cengage Learning. All rights reserved 2

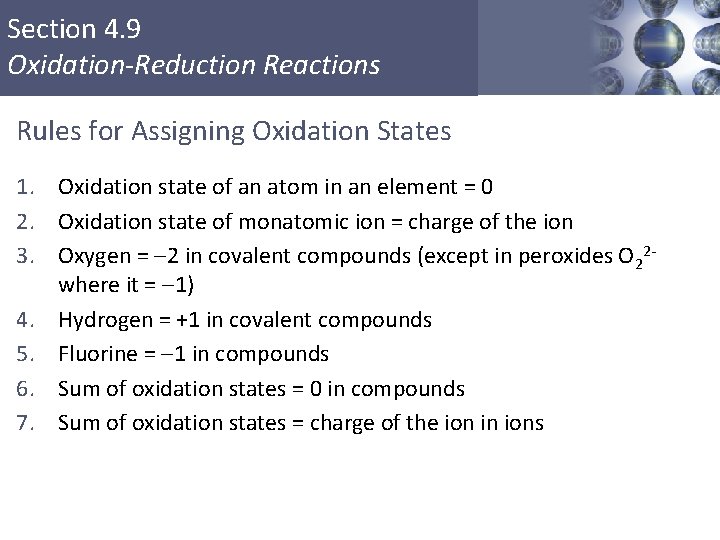
Section 4. 9 Oxidation-Reduction Reactions Rules for Assigning Oxidation States 1. Oxidation state of an atom in an element = 0 2. Oxidation state of monatomic ion = charge of the ion 3. Oxygen = -2 in covalent compounds (except in peroxides O 22 where it = -1) 4. Hydrogen = +1 in covalent compounds 5. Fluorine = -1 in compounds 6. Sum of oxidation states = 0 in compounds 7. Sum of oxidation states = charge of the ion in ions Copyright © Cengage Learning. All rights reserved 3

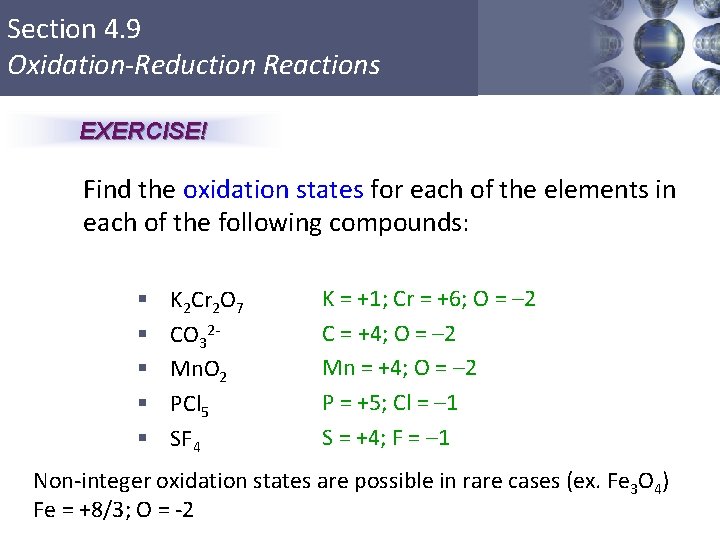
Section 4. 9 Oxidation-Reduction Reactions EXERCISE! Find the oxidation states for each of the elements in each of the following compounds: § § § K 2 Cr 2 O 7 CO 32 Mn. O 2 PCl 5 SF 4 K = +1; Cr = +6; O = – 2 C = +4; O = – 2 Mn = +4; O = – 2 P = +5; Cl = – 1 S = +4; F = – 1 Non-integer oxidation states are possible in rare cases (ex. Fe 3 O 4) Fe = +8/3; O = -2 Copyright © Cengage Learning. All rights reserved 4

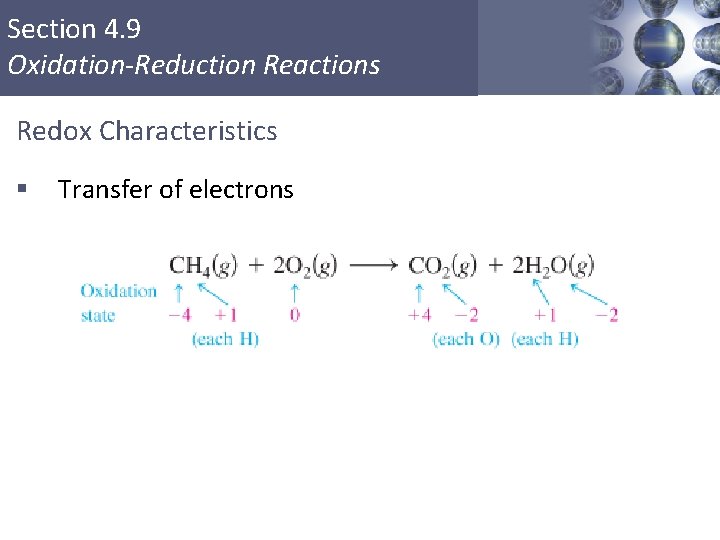
Section 4. 9 Oxidation-Reduction Reactions Redox Characteristics § Transfer of electrons Copyright © Cengage Learning. All rights reserved 5

Section 4. 9 Oxidation-Reduction Reactions Redox Characteristics § § Transfer of electrons Transfer may occur to form ions § Oxidation – increase in oxidation state (loss of electrons); reducing agent (reductant) Reduction – decrease in oxidation state (gain of electrons); oxidizing agent (oxidant) § Copyright © Cengage Learning. All rights reserved 6

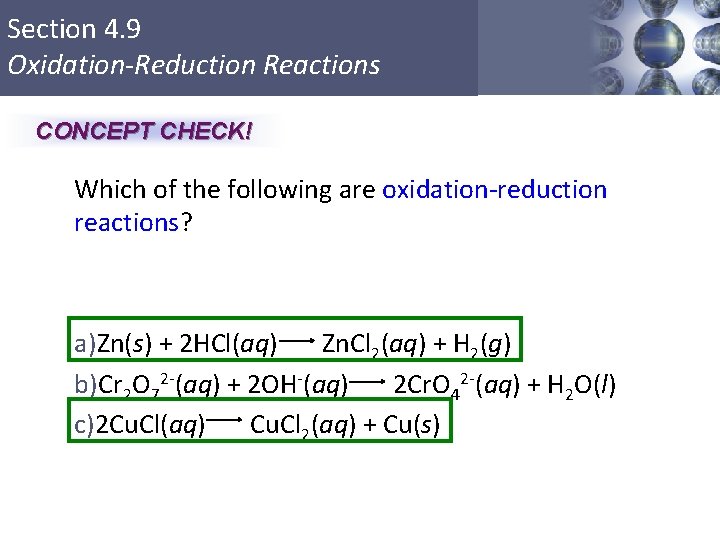
Section 4. 9 Oxidation-Reduction Reactions CONCEPT CHECK! Which of the following are oxidation-reduction reactions? a)Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) b)Cr 2 O 72 -(aq) + 2 OH-(aq) 2 Cr. O 42 -(aq) + H 2 O(l) c)2 Cu. Cl(aq) Cu. Cl 2(aq) + Cu(s) Copyright © Cengage Learning. All rights reserved

Section 4. 9 Oxidation-Reduction Reactions Balance an equation showing the oxidation of Fe 2+ to Fe 3+ by dichromate ions in an acidic medium. As a result, the Cr 2 O 72 - ions are reduced to Cr 3+ ions. Write the unbalanced equation for the reaction in ionic form.

Section 4. 9 Oxidation-Reduction Reactions Separate the equations into two half reactions.

Section 4. 9 Oxidation-Reduction Reactions Balance each half- reaction for the number and types of atoms and charges. For reactions in an acidic medium, add H 2 O to balance the O atoms and H+ to balance the H atoms.

Section 4. 9 Oxidation-Reduction Reactions Balance each half- reaction for the number and types of atoms and charges. For reactions in an acidic medium, add H 2 O to balance the O atoms and H+ to balance the H atoms.

Section 4. 9 Oxidation-Reduction Reactions Add the two half- equations together and balance the final equation by inspection. The electrons on both sides must cancel. If the oxidation and reduction half-reactions contain different numbers of electrons, we need to multiply one or both to equalize the number of electrons.

Section 4. 9 Oxidation-Reduction Reactions Verify that the equations contains the same type and numbers of atoms and the same charges on both sides of the equation.

Section 4. 9 Oxidation-Reduction Reactions If the reaction occurs in a basic medium, we proceed through step 4 as above. Then, for every H+ ion we add an equal number of OH- to both sides of the equation. Where H+ and OH- appear on the same side of the equation, we combine the ions to give H 2 O.

Section 4. 9 Oxidation-Reduction Reactions § Write a balanced ionic equation to represent the oxidation of iodide ion (I-) by permanganate ion (Mn. O 4 -) in basic solution to yield molecular iodine (I 2) and manganese (IV) oxide (Mn. O 2).