Section 4 7Light Matter Electrons Absorbing Energy Photon
Section 4. 7—Light & Matter
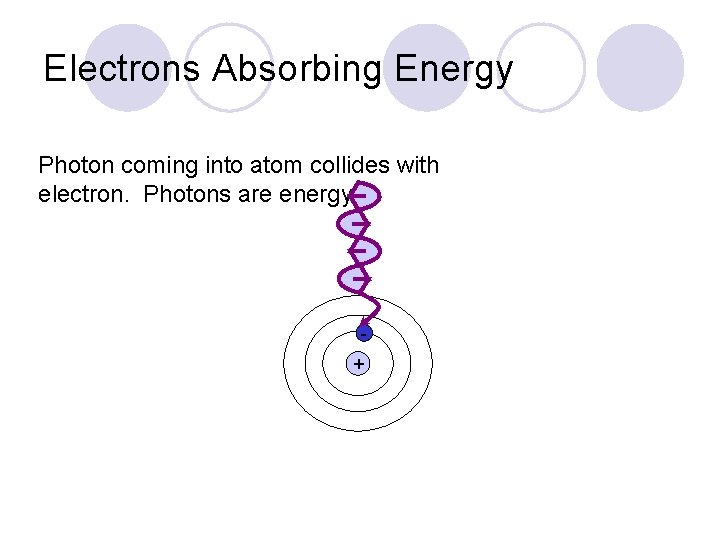
Electrons Absorbing Energy Photon coming into atom collides with electron. Photons are energy. +
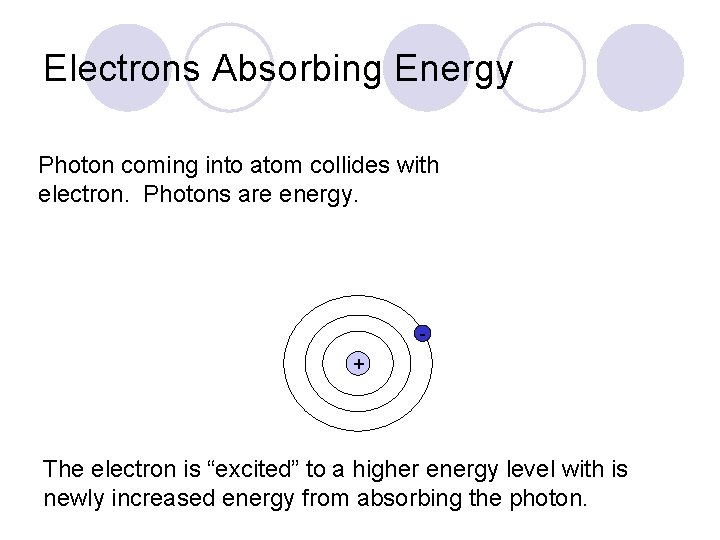
Electrons Absorbing Energy Photon coming into atom collides with electron. Photons are energy. + The electron is “excited” to a higher energy level with is newly increased energy from absorbing the photon.
Excitation l The process of an electron absorbing a photon of light (energy) and being promoted to a higher energy level from its “ground state”
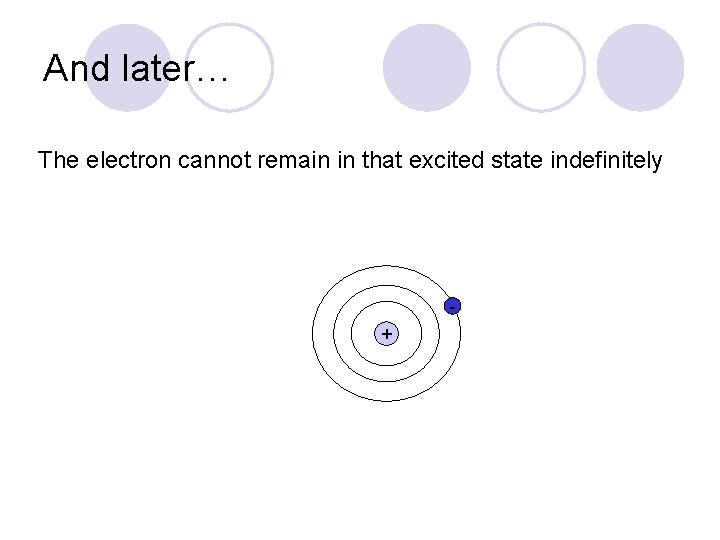
And later… The electron cannot remain in that excited state indefinitely +
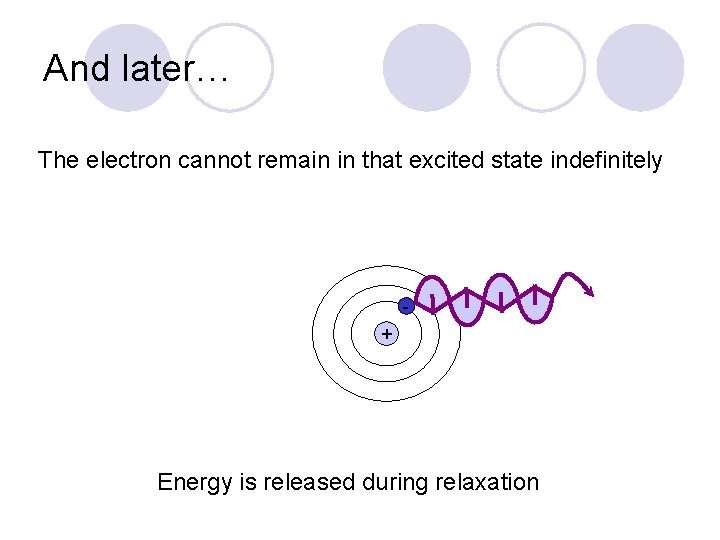
And later… The electron cannot remain in that excited state indefinitely + Energy is released during relaxation

Relaxation l The process of an electron releasing a photon of light (energy) and falling back down to a lower energy level.

Energy of photon and levels jumped l The higher the energy of the photon, the greater the electron jump! l A photon of UV light has more energy than a photon of Infrared light ¡ The UV photon would cause a higher energy jump (jump up more levels) than the IR photon.

Total energy in = Total energy out l However much energy was absorbed must be released again, but it can be released in smaller packets l A high energy photon might be absorbed, but two lower energy photons might be released as the electron falls in a “stepwise” manner.
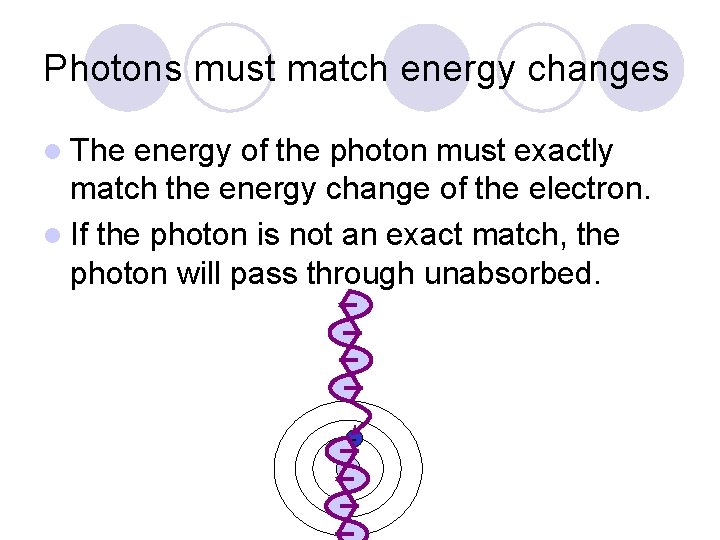
Photons must match energy changes l The energy of the photon must exactly match the energy change of the electron. l If the photon is not an exact match, the photon will pass through unabsorbed. +
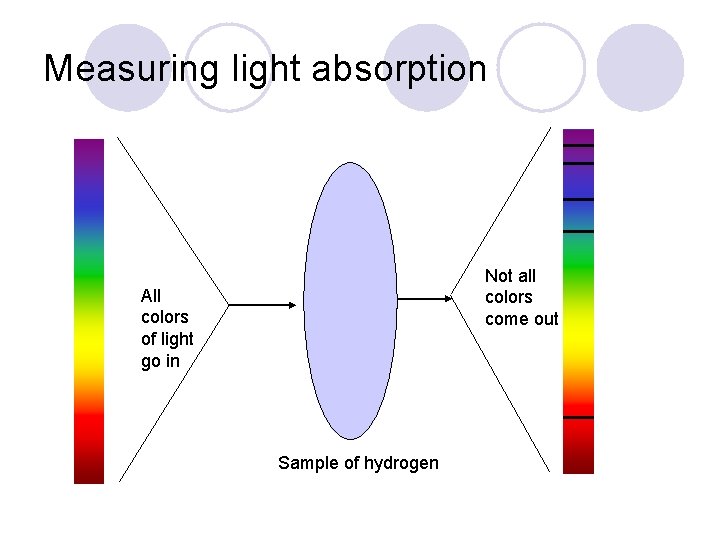
Measuring light absorption Not all colors come out All colors of light go in Sample of hydrogen
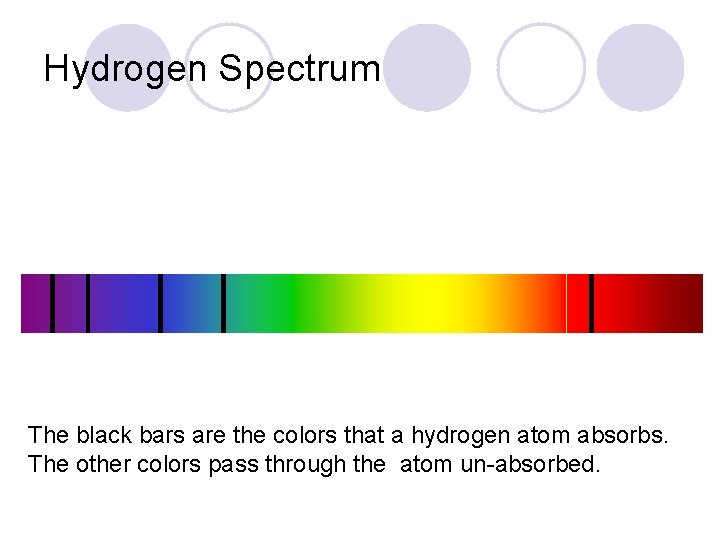
Hydrogen Spectrum The black bars are the colors that a hydrogen atom absorbs. The other colors pass through the atom un-absorbed.
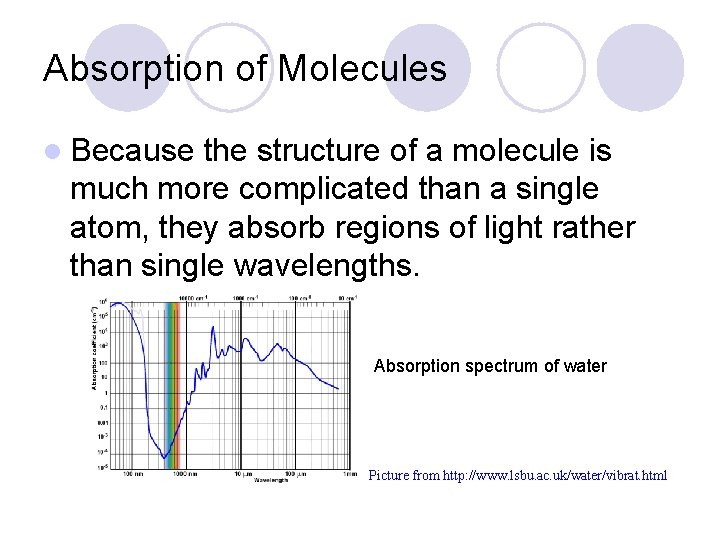
Absorption of Molecules l Because the structure of a molecule is much more complicated than a single atom, they absorb regions of light rather than single wavelengths. Absorption spectrum of water Picture from http: //www. lsbu. ac. uk/water/vibrat. html

Ways of producing light l Fluorescence: visible light is absorbed and visible light is emitted at the same time— the relaxation happens very quickly after excitation l Phosphorescence: Visible light is absorbed and then a while later is emitted— relaxation occurs after a period of time
Ways of producing light Incandescence: Energy is put in from heat and given off as visible light l Chemiluminescence: Energy released during a chemical reaction is absorbed to cause excitation. Relaxation produces visible light l Biolouminescence: Chemiluminescence that occurs in a biological organism. l Triboluminescence: Physical pressure or torque provides energy for excitation. Relaxation produces visible light. l

What did you learn about glowing things?
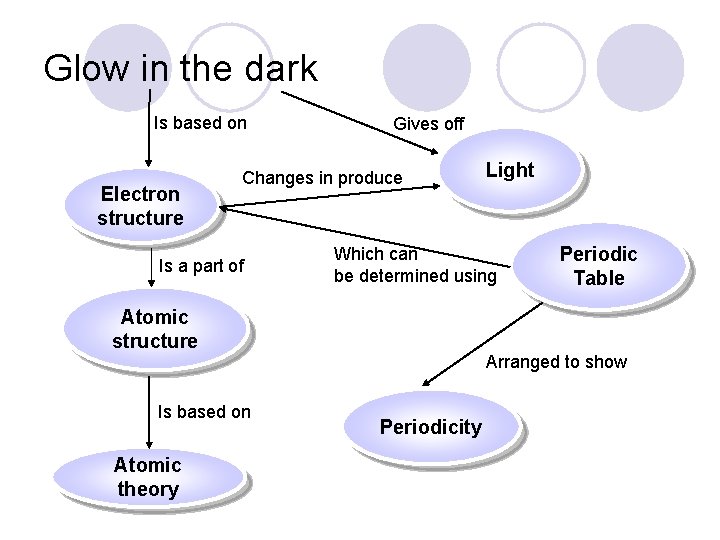
Glow in the dark Is based on Electron structure Gives off Changes in produce Is a part of Which can be determined using Atomic structure Is based on Atomic theory Light Periodic Table Arranged to show Periodicity
- Slides: 17