Section 3 The Quantum Mechanical Model of the




- Slides: 19

Section 3: The Quantum Mechanical Model of the Atom


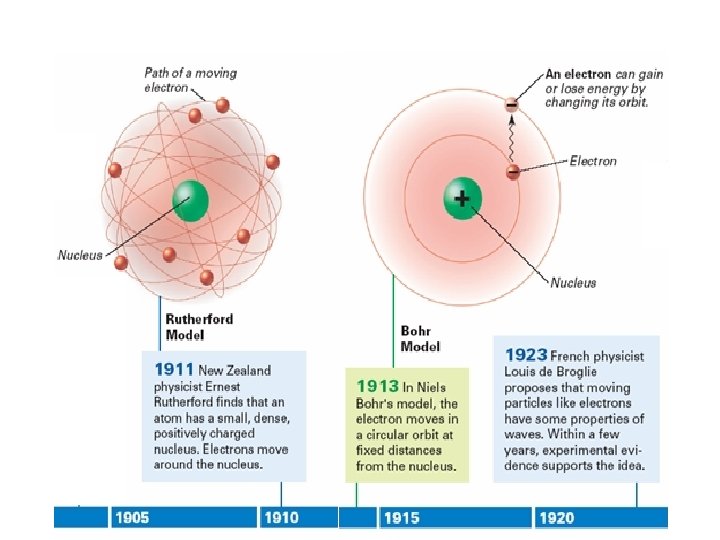
Bohr's Model of the Atom (cont. ) • Bohr’s model explained the hydrogen’s spectral lines, but failed to explain any other element’s lines. • The behavior of electrons is still not fully understood, but it is known they do not move around the nucleus in circular orbits.

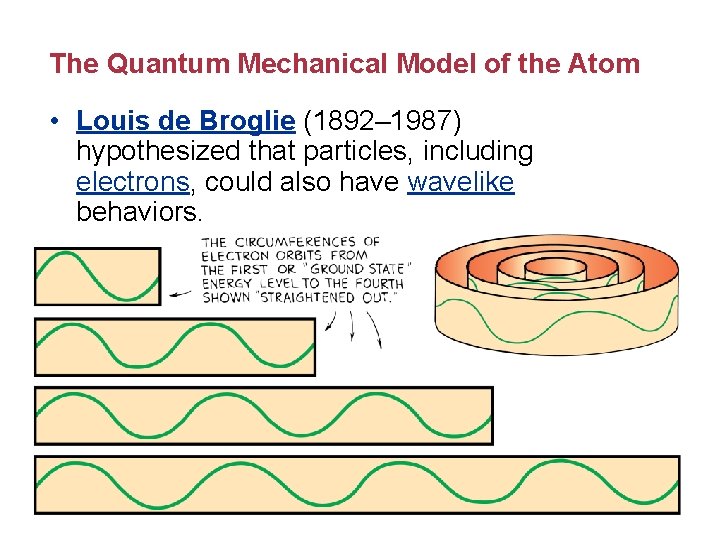
The Quantum Mechanical Model of the Atom • Louis de Broglie (1892– 1987) hypothesized that particles, including electrons, could also have wavelike behaviors.

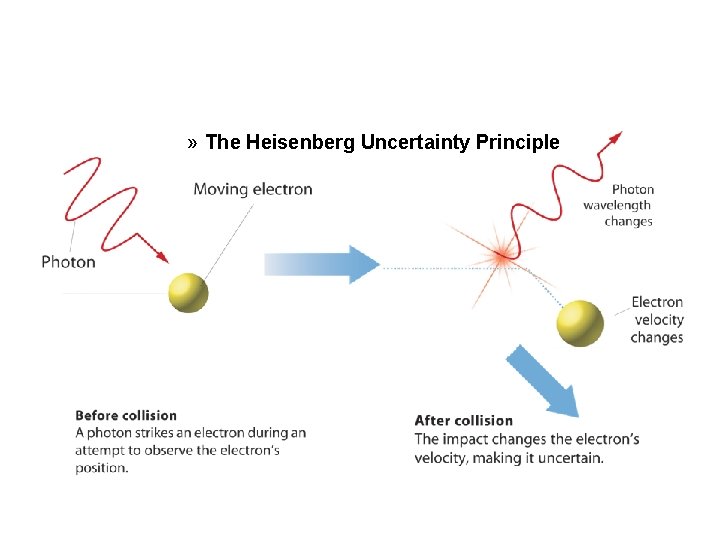
The Quantum Mechanical Model of the Atom (cont. ) • Heisenberg showed it is impossible to take any measurement of an object without disturbing it. • The Heisenberg uncertainty principle states that it is fundamentally impossible to know precisely both the velocity and position of a particle at the same time. • The only quantity that can be known is the probability for an electron to occupy a certain region around the nucleus.

» The Heisenberg Uncertainty Principle

The Quantum Mechanical Model of the Atom (cont. ) • Schrödinger treated electrons as waves in a model called the quantum mechanical model of the atom. • Schrödinger’s equation applied equally well to elements other than hydrogen.

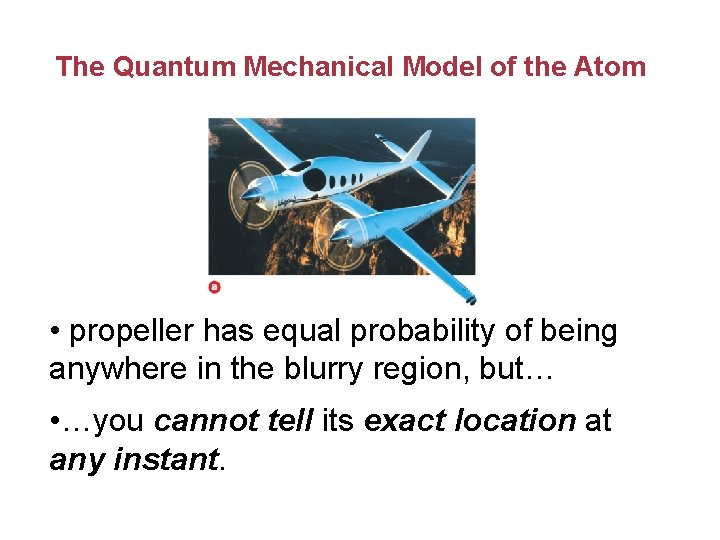
The Quantum Mechanical Model of the Atom • propeller has equal probability of being anywhere in the blurry region, but… • …you cannot tell its exact location at any instant.

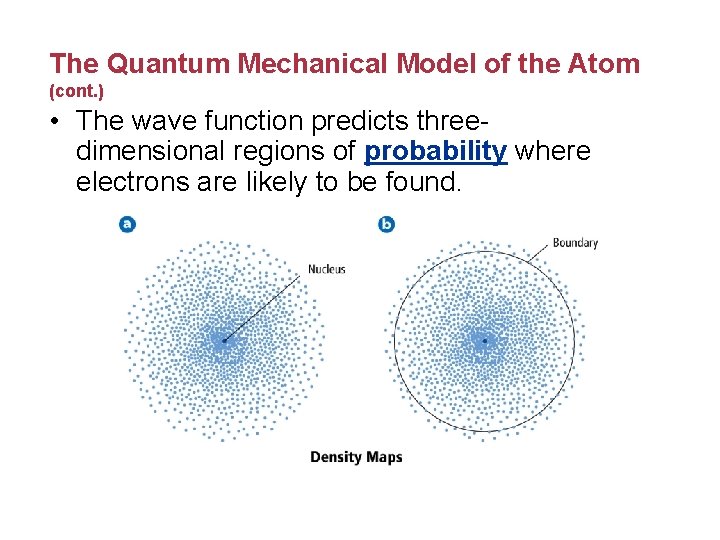
The Quantum Mechanical Model of the Atom (cont. ) • The wave function predicts threedimensional regions of probability where electrons are likely to be found.

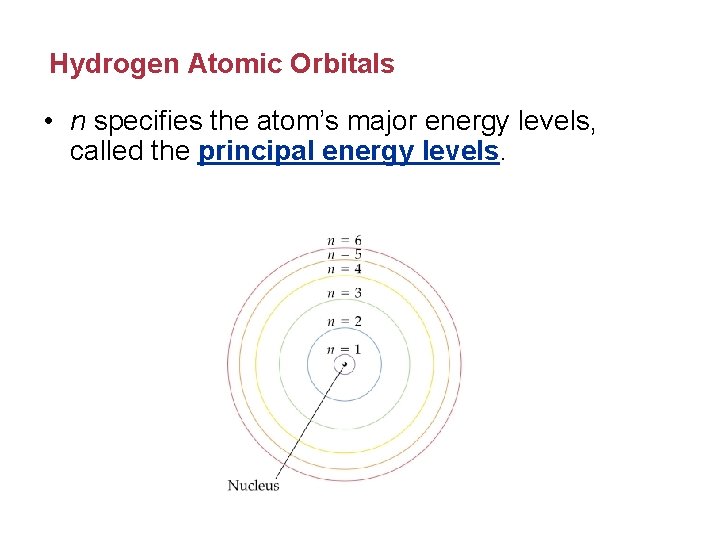
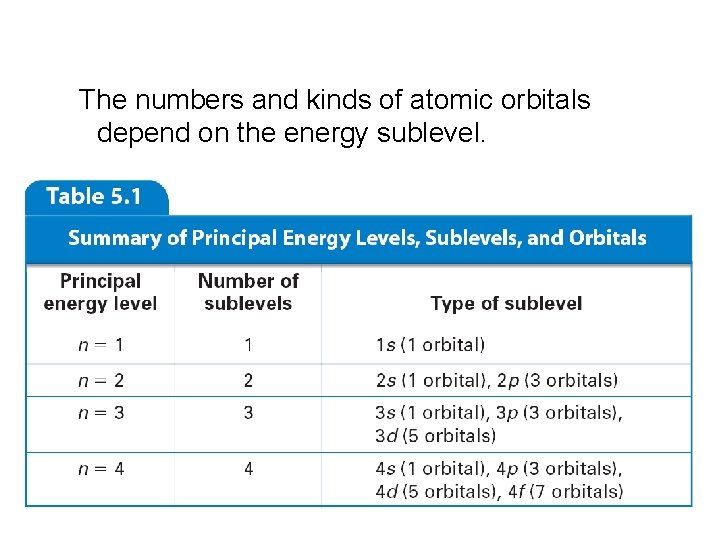
Hydrogen Atomic Orbitals • n specifies the atom’s major energy levels, called the principal energy levels.

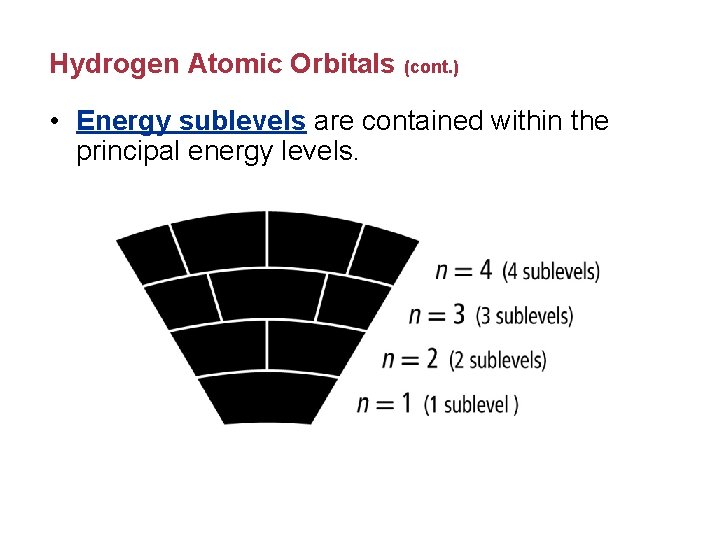
Hydrogen Atomic Orbitals (cont. ) • Energy sublevels are contained within the principal energy levels.

Hydrogen Atomic Orbitals (cont. ) • Each energy sublevel relates to orbitals of different shape. Atomic Orbitals are 3 -dimensional regions with a high probability of electrons

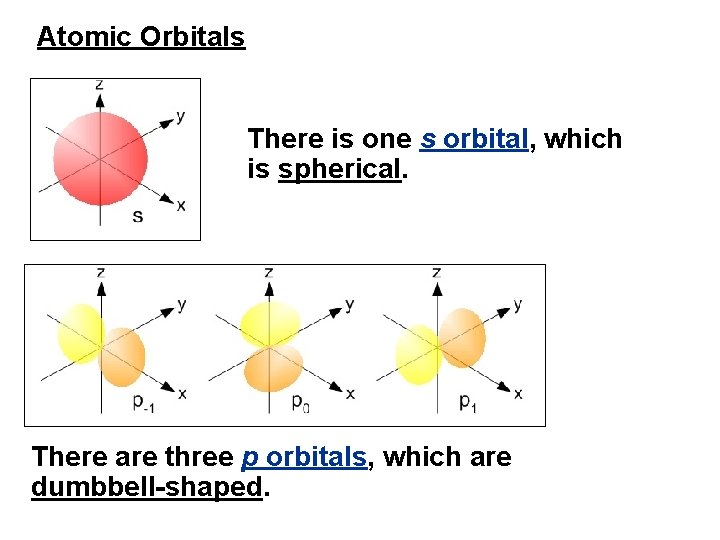
Atomic Orbitals There is one s orbital, which is spherical. There are three p orbitals, which are dumbbell-shaped.

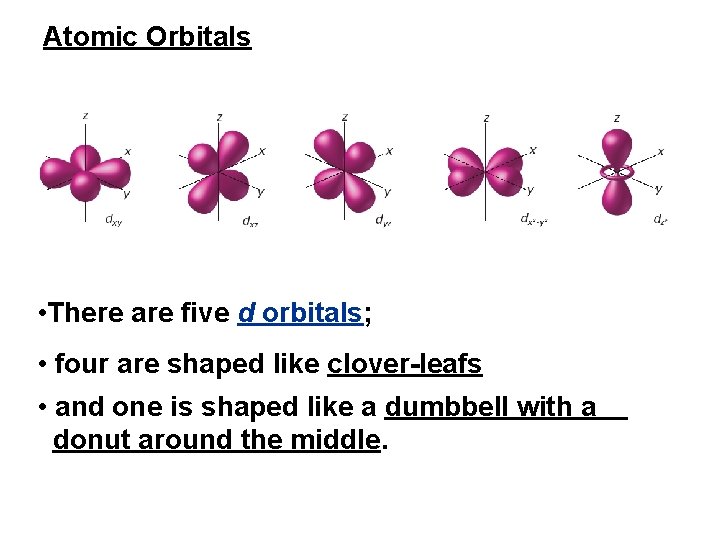
Atomic Orbitals • There are five d orbitals; • four are shaped like clover-leafs • and one is shaped like a dumbbell with a donut around the middle.

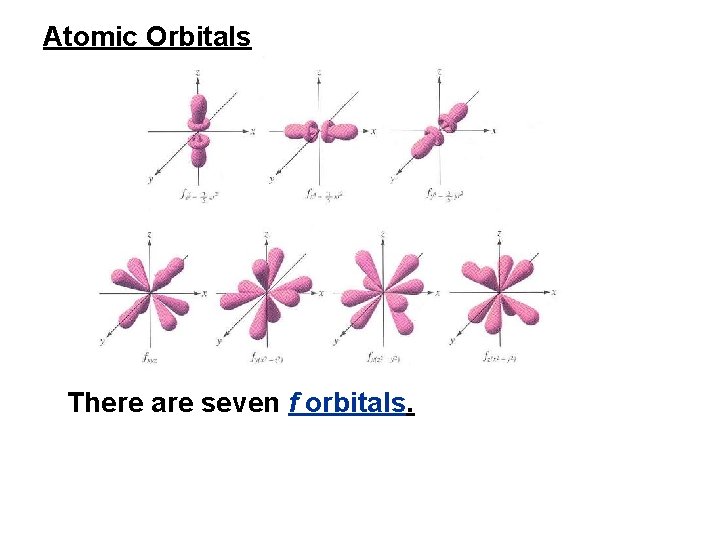
• 5. Atomic Orbitals 1 There are seven f orbitals.

The numbers and kinds of atomic orbitals depend on the energy sublevel.

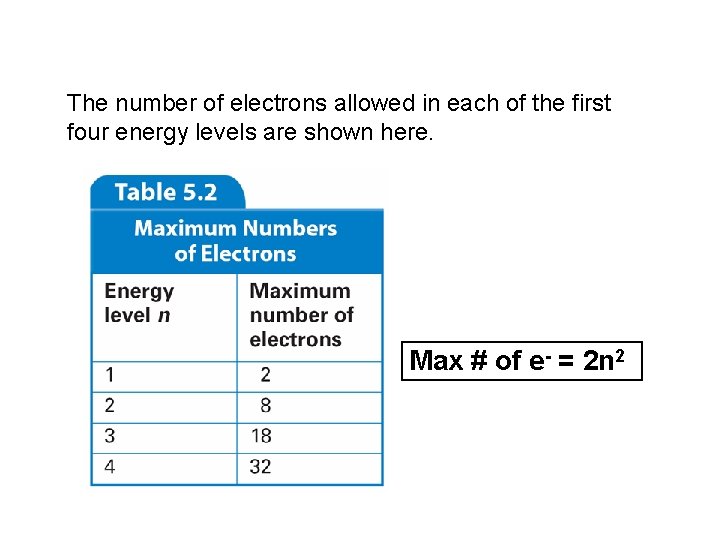
The number of electrons allowed in each of the first four energy levels are shown here. Max # of e- = 2 n 2

Section 5. 2 Assessment Which atomic suborbitals have a “dumbbell” shape? A. s B. f C. p D. d A. B. C. D. A B C D

Section 5. 2 Assessment Who proposed that particles could also exhibit wavelike behaviors? A. Bohr B. Einstein C. Rutherford D. de Broglie A. B. C. D. A B C D