Section 3 Limiting Reactants A chemical reaction stops


- Slides: 15

Section 3: Limiting Reactants A chemical reaction stops when one of the reactants is used up. K What I Know W What I Want to Find Out L What I Learned

• 8(E) Perform stoichiometric calculations, including determination of mass relationships between reactants and products, calculation of limiting reagents, and percent yield. • 8(A) Define and use the concept of a mole. • 8(D) Use the law of conservation of mass to write and balance chemical equations. • 2(G) Express and manipulate chemical quantities using scientific conventions and mathematical procedures, including dimensional analysis, scientific notation, and significant figures. Copyright © Mc. Graw-Hill Education Limiting Reactants

Essential Questions • In a chemical reaction, which reactant is the limiting reactant? • How do you calculate the masses of product and excess reactant when the amounts of more than one reactant are given? Copyright © Mc. Graw-Hill Education Limiting Reactants

Vocabulary Review New • molar mass • limiting reactant • excess reactant Copyright © Mc. Graw-Hill Education Limiting Reactants

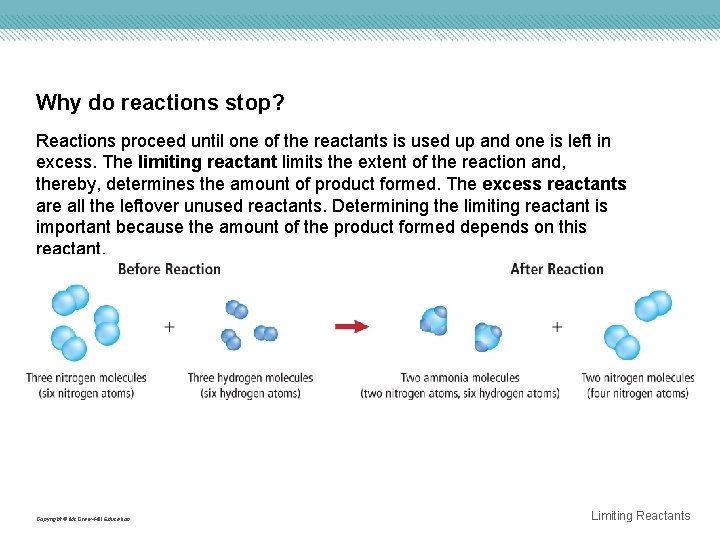
Why do reactions stop? Reactions proceed until one of the reactants is used up and one is left in excess. The limiting reactant limits the extent of the reaction and, thereby, determines the amount of product formed. The excess reactants are all the leftover unused reactants. Determining the limiting reactant is important because the amount of the product formed depends on this reactant. Copyright © Mc. Graw-Hill Education Limiting Reactants

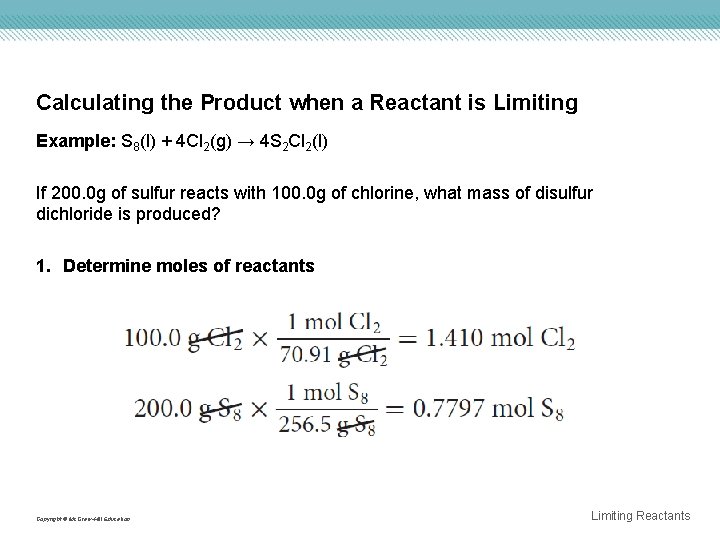
Calculating the Product when a Reactant is Limiting Example: S 8(l) + 4 Cl 2(g) → 4 S 2 Cl 2(l) If 200. 0 g of sulfur reacts with 100. 0 g of chlorine, what mass of disulfur dichloride is produced? 1. Determine moles of reactants Copyright © Mc. Graw-Hill Education Limiting Reactants

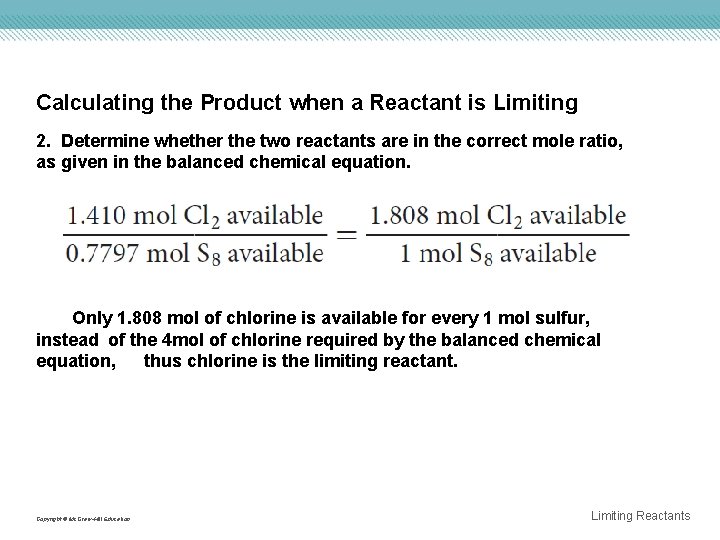
Calculating the Product when a Reactant is Limiting 2. Determine whether the two reactants are in the correct mole ratio, as given in the balanced chemical equation. Only 1. 808 mol of chlorine is available for every 1 mol sulfur, instead of the 4 mol of chlorine required by the balanced chemical equation, thus chlorine is the limiting reactant. Copyright © Mc. Graw-Hill Education Limiting Reactants

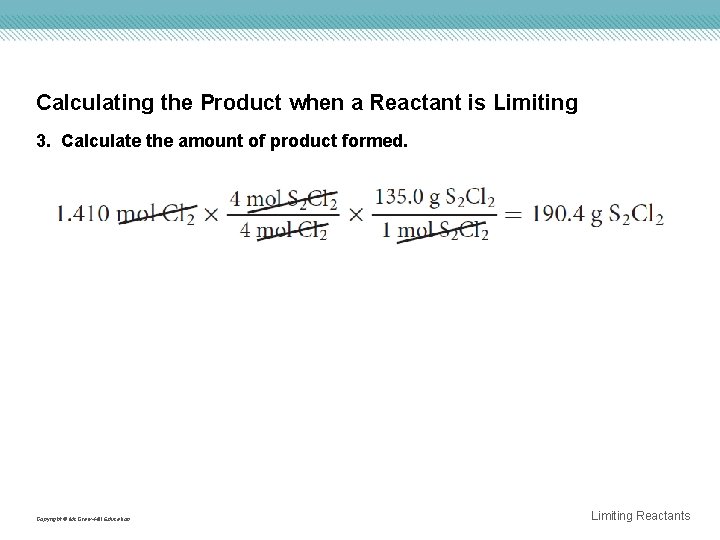
Calculating the Product when a Reactant is Limiting 3. Calculate the amount of product formed. Copyright © Mc. Graw-Hill Education Limiting Reactants

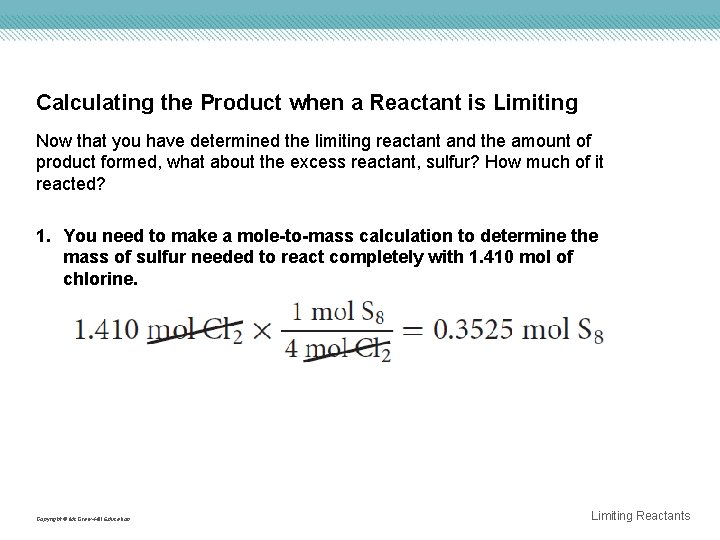
Calculating the Product when a Reactant is Limiting Now that you have determined the limiting reactant and the amount of product formed, what about the excess reactant, sulfur? How much of it reacted? 1. You need to make a mole-to-mass calculation to determine the mass of sulfur needed to react completely with 1. 410 mol of chlorine. Copyright © Mc. Graw-Hill Education Limiting Reactants

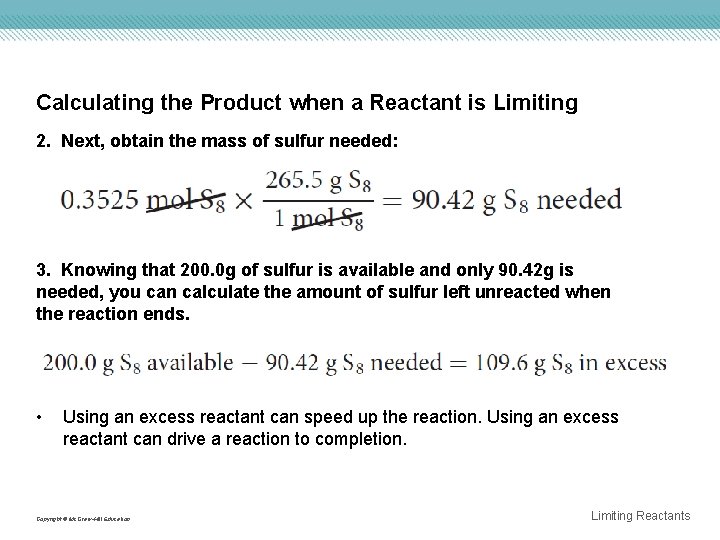
Calculating the Product when a Reactant is Limiting 2. Next, obtain the mass of sulfur needed: 3. Knowing that 200. 0 g of sulfur is available and only 90. 42 g is needed, you can calculate the amount of sulfur left unreacted when the reaction ends. • Using an excess reactant can speed up the reaction. Using an excess reactant can drive a reaction to completion. Copyright © Mc. Graw-Hill Education Limiting Reactants

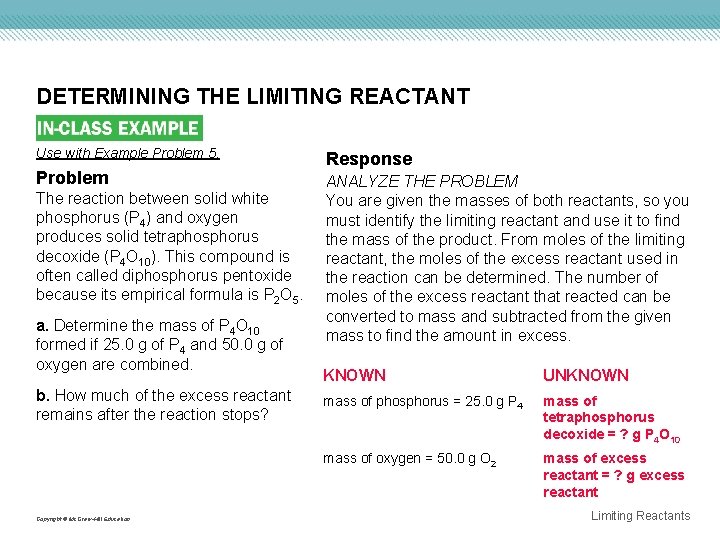
DETERMINING THE LIMITING REACTANT Use with Example Problem 5. Problem The reaction between solid white phosphorus (P 4) and oxygen produces solid tetraphosphorus decoxide (P 4 O 10). This compound is often called diphosphorus pentoxide because its empirical formula is P 2 O 5. a. Determine the mass of P 4 O 10 formed if 25. 0 g of P 4 and 50. 0 g of oxygen are combined. b. How much of the excess reactant remains after the reaction stops? Copyright © Mc. Graw-Hill Education Response ANALYZE THE PROBLEM You are given the masses of both reactants, so you must identify the limiting reactant and use it to find the mass of the product. From moles of the limiting reactant, the moles of the excess reactant used in the reaction can be determined. The number of moles of the excess reactant that reacted can be converted to mass and subtracted from the given mass to find the amount in excess. KNOWN UNKNOWN mass of phosphorus = 25. 0 g P 4 mass of tetraphosphorus decoxide = ? g P 4 O 10 mass of oxygen = 50. 0 g O 2 mass of excess reactant = ? g excess reactant Limiting Reactants

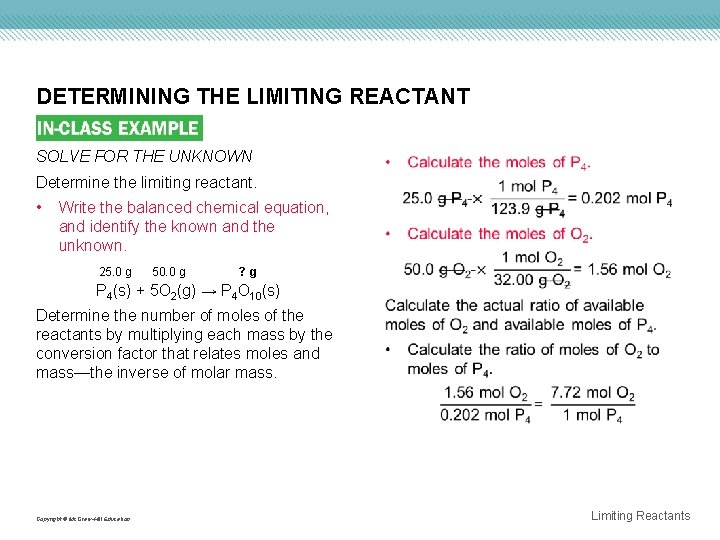
DETERMINING THE LIMITING REACTANT SOLVE FOR THE UNKNOWN Determine the limiting reactant. • Write the balanced chemical equation, and identify the known and the unknown. 25. 0 g 50. 0 g ? g P 4(s) + 5 O 2(g) → P 4 O 10(s) Determine the number of moles of the reactants by multiplying each mass by the conversion factor that relates moles and mass—the inverse of molar mass. Copyright © Mc. Graw-Hill Education Limiting Reactants

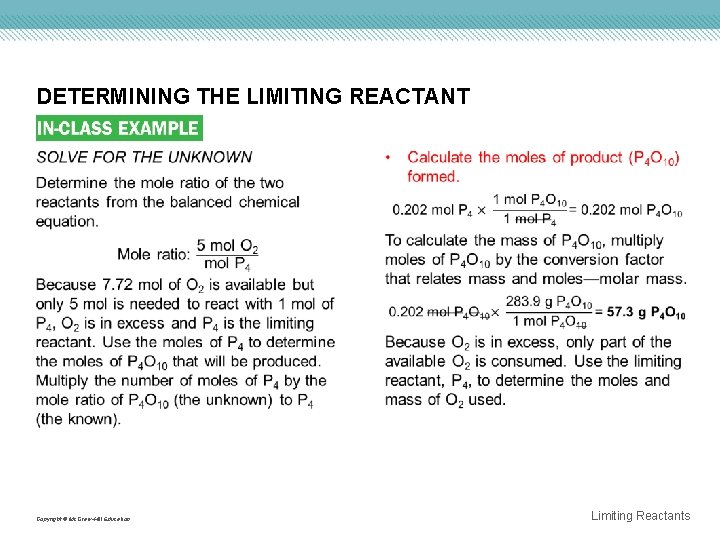
DETERMINING THE LIMITING REACTANT Copyright © Mc. Graw-Hill Education Limiting Reactants

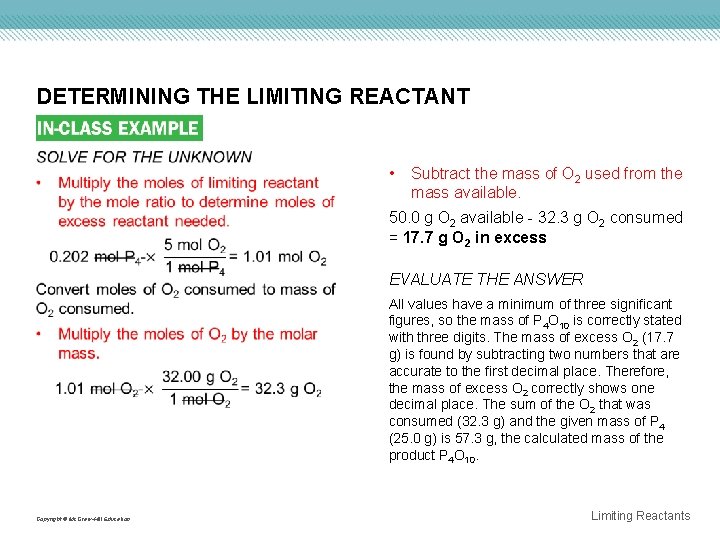
DETERMINING THE LIMITING REACTANT • Subtract the mass of O 2 used from the mass available. 50. 0 g O 2 available - 32. 3 g O 2 consumed = 17. 7 g O 2 in excess EVALUATE THE ANSWER All values have a minimum of three significant figures, so the mass of P 4 O 10 is correctly stated with three digits. The mass of excess O 2 (17. 7 g) is found by subtracting two numbers that are accurate to the first decimal place. Therefore, the mass of excess O 2 correctly shows one decimal place. The sum of the O 2 that was consumed (32. 3 g) and the given mass of P 4 (25. 0 g) is 57. 3 g, the calculated mass of the product P 4 O 10. Copyright © Mc. Graw-Hill Education Limiting Reactants

Review Essential Questions • In a chemical reaction, which reactant is the limiting reactant? • How do you calculate the masses of product and excess reactant when the amounts of more than one reactant are given? Vocabulary • limiting reactant Copyright © Mc. Graw-Hill Education • excess reactant Limiting Reactants