Section 3 Ethers Thiols Sulfides Amines Ethers ROR

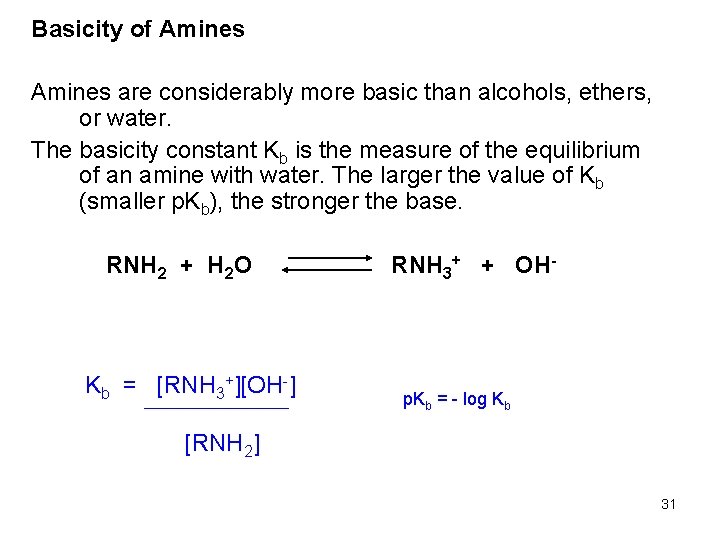
- Slides: 38

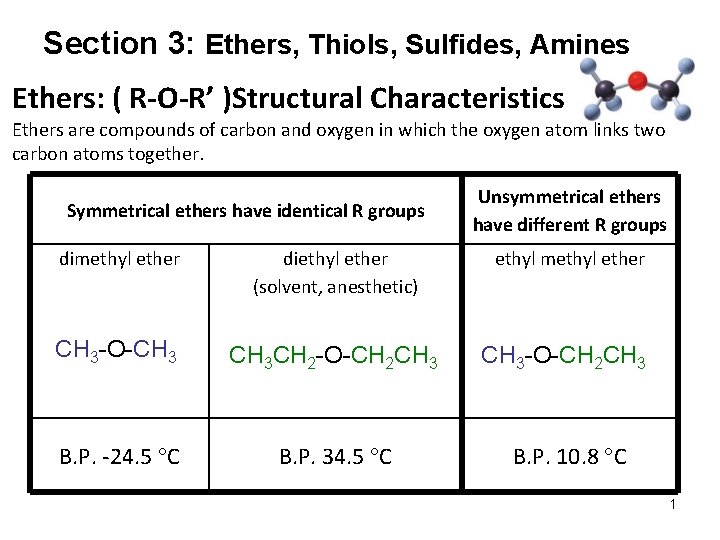
Section 3: Ethers, Thiols, Sulfides, Amines Ethers: ( R-O-R’ )Structural Characteristics Ethers are compounds of carbon and oxygen in which the oxygen atom links two carbon atoms together. Symmetrical ethers have identical R groups Unsymmetrical ethers have different R groups dimethyl ether diethyl ether (solvent, anesthetic) ethyl methyl ether CH 3 -O-CH 3 CH 2 -O-CH 2 CH 3 -O-CH 2 CH 3 B. P. -24. 5 C B. P. 34. 5 C B. P. 10. 8 C 1

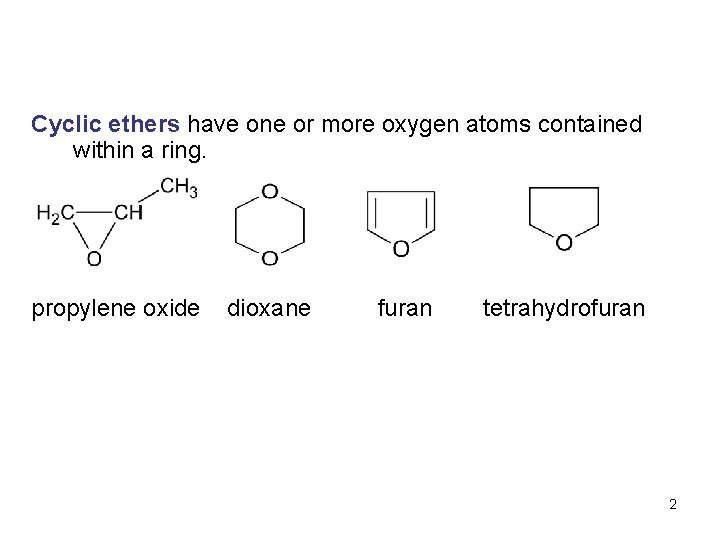
Cyclic ethers have one or more oxygen atoms contained within a ring. propylene oxide dioxane furan tetrahydrofuran 2

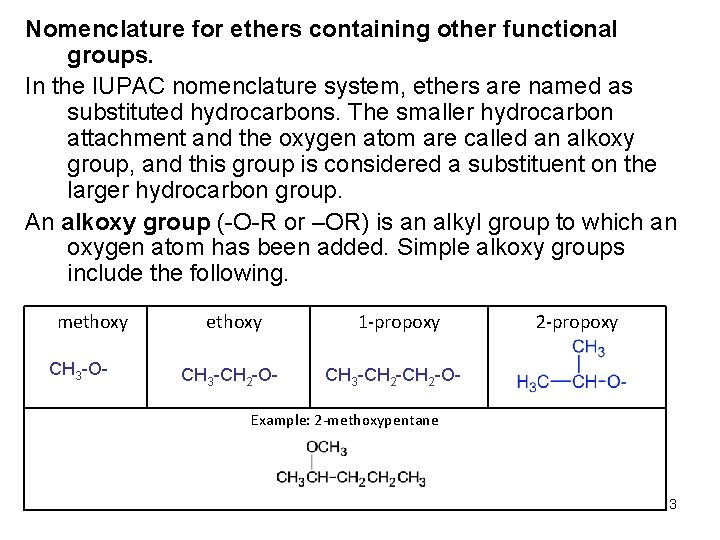
Nomenclature for ethers containing other functional groups. In the IUPAC nomenclature system, ethers are named as substituted hydrocarbons. The smaller hydrocarbon attachment and the oxygen atom are called an alkoxy group, and this group is considered a substituent on the larger hydrocarbon group. An alkoxy group (-O-R or –OR) is an alkyl group to which an oxygen atom has been added. Simple alkoxy groups include the following. methoxy CH 3 -O- ethoxy CH 3 -CH 2 -O- 1 -propoxy 2 -propoxy CH 3 -CH 2 -O- Example: 2 -methoxypentane 3

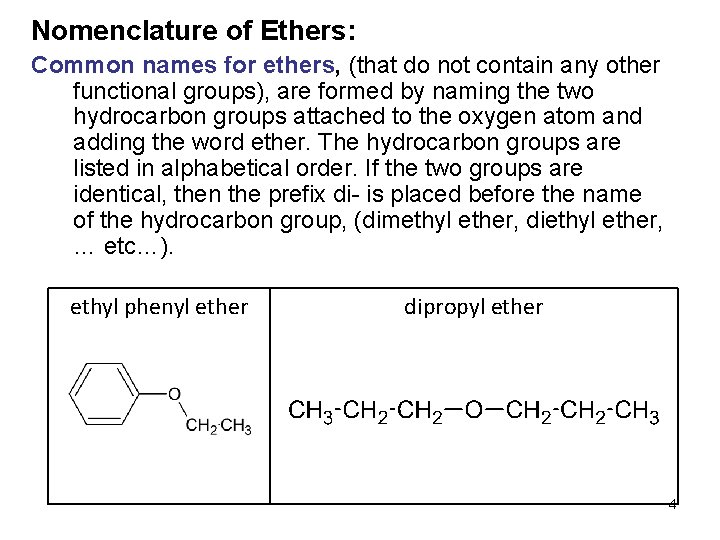
Nomenclature of Ethers: Common names for ethers, (that do not contain any other functional groups), are formed by naming the two hydrocarbon groups attached to the oxygen atom and adding the word ether. The hydrocarbon groups are listed in alphabetical order. If the two groups are identical, then the prefix di- is placed before the name of the hydrocarbon group, (dimethyl ether, diethyl ether, … etc…). ethyl phenyl ether dipropyl ether 4

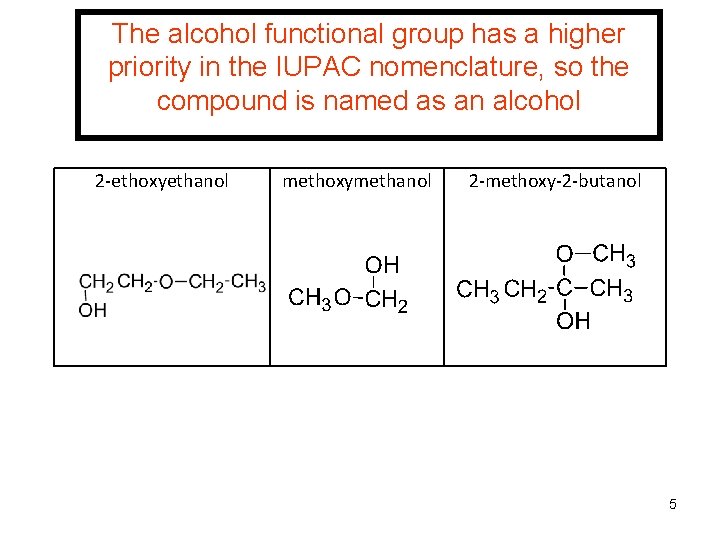
The alcohol functional group has a higher priority in the IUPAC nomenclature, so the compound is named as an alcohol 2 -ethoxyethanol methoxymethanol 2 -methoxy-2 -butanol 5

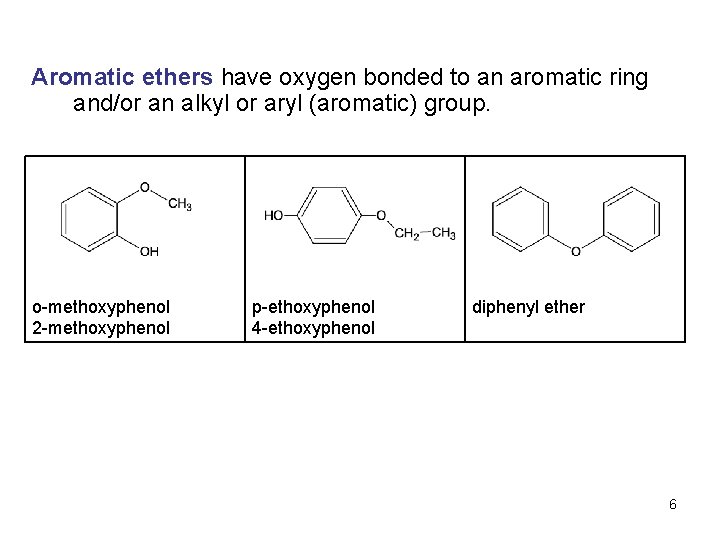
Aromatic ethers have oxygen bonded to an aromatic ring and/or an alkyl or aryl (aromatic) group. o-methoxyphenol 2 -methoxyphenol p-ethoxyphenol 4 -ethoxyphenol diphenyl ether 6

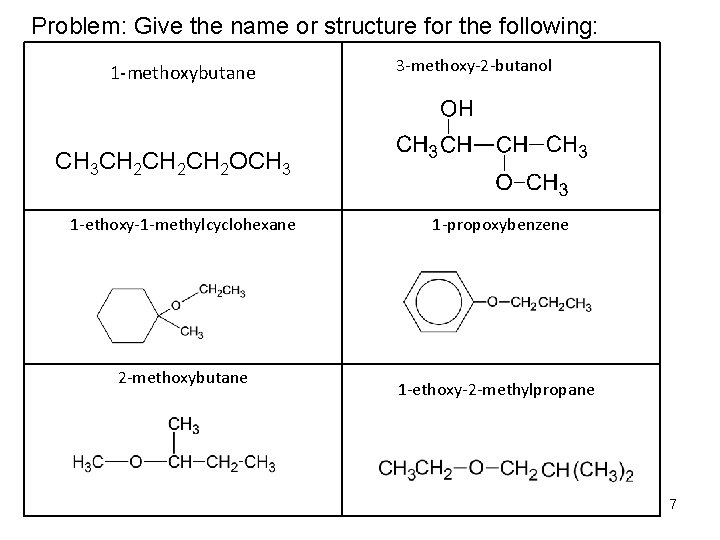
Problem: Give the name or structure for the following: 1 -methoxybutane 3 -methoxy-2 -butanol CH 3 CH 2 CH 2 OCH 3 1 -ethoxy-1 -methylcyclohexane 2 -methoxybutane 1 -propoxybenzene 1 -ethoxy-2 -methylpropane 7

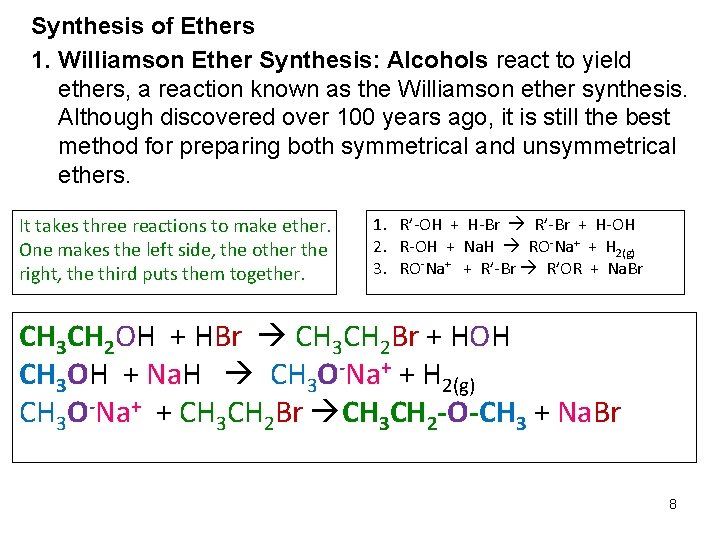
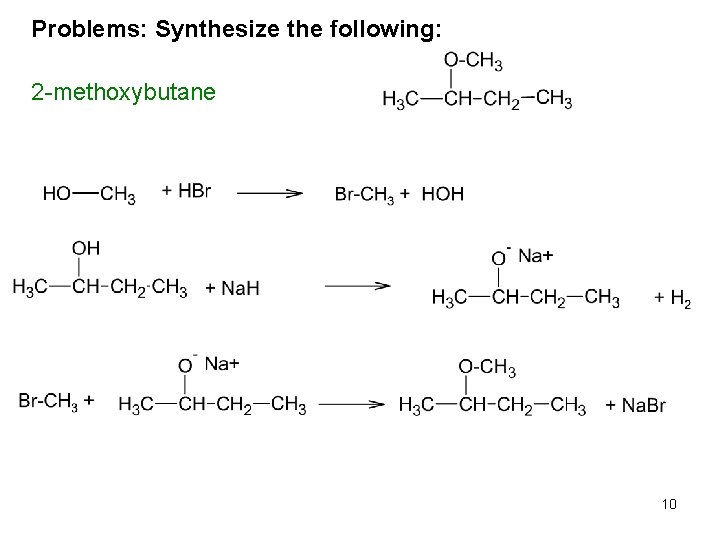
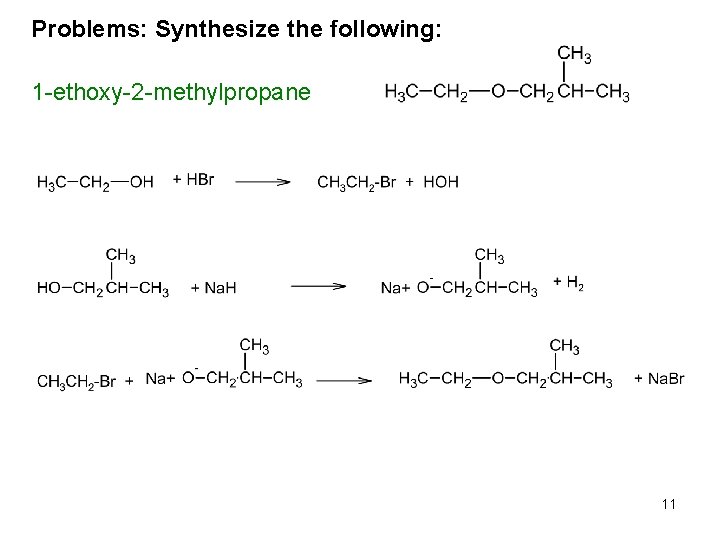
Synthesis of Ethers 1. Williamson Ether Synthesis: Alcohols react to yield ethers, a reaction known as the Williamson ether synthesis. Although discovered over 100 years ago, it is still the best method for preparing both symmetrical and unsymmetrical ethers. It takes three reactions to make ether. One makes the left side, the other the right, the third puts them together. 1. R’-OH + H-Br R’-Br + H-OH 2. R-OH + Na. H RO-Na+ + H 2(g) 3. RO-Na+ + R’-Br R’OR + Na. Br CH 3 CH 2 OH + HBr CH 3 CH 2 Br + HOH CH 3 OH + Na. H CH 3 O-Na+ + H 2(g) CH 3 O-Na+ + CH 3 CH 2 Br CH 3 CH 2 -O-CH 3 + Na. Br 8

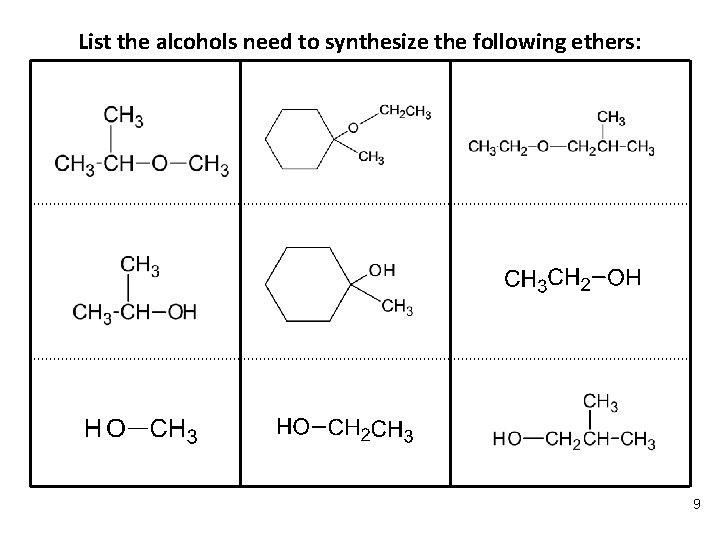
List the alcohols need to synthesize the following ethers: 9

Problems: Synthesize the following: 2 -methoxybutane 10

Problems: Synthesize the following: 1 -ethoxy-2 -methylpropane 11

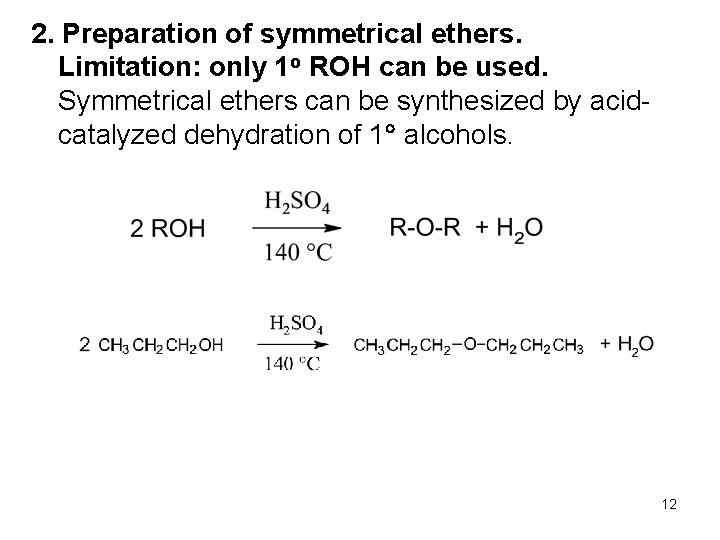
2. Preparation of symmetrical ethers. Limitation: only 1 o ROH can be used. Symmetrical ethers can be synthesized by acidcatalyzed dehydration of 1 alcohols. 12

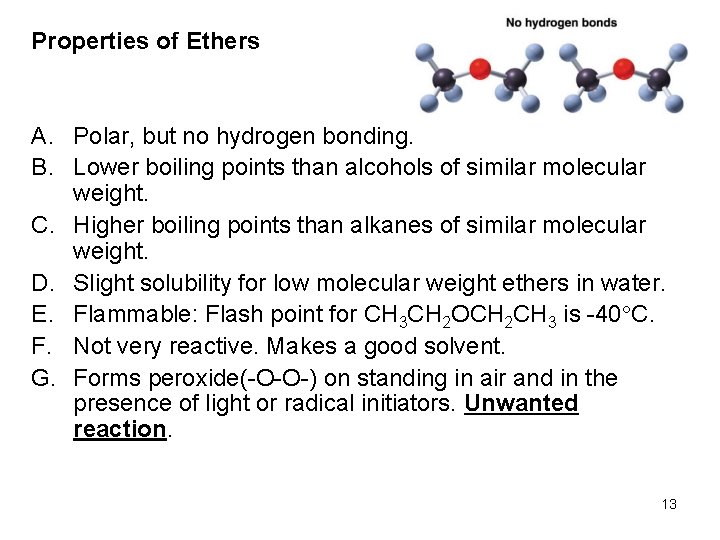
Properties of Ethers A. Polar, but no hydrogen bonding. B. Lower boiling points than alcohols of similar molecular weight. C. Higher boiling points than alkanes of similar molecular weight. D. Slight solubility for low molecular weight ethers in water. E. Flammable: Flash point for CH 3 CH 2 OCH 2 CH 3 is -40 C. F. Not very reactive. Makes a good solvent. G. Forms peroxide(-O-O-) on standing in air and in the presence of light or radical initiators. Unwanted reaction. 13

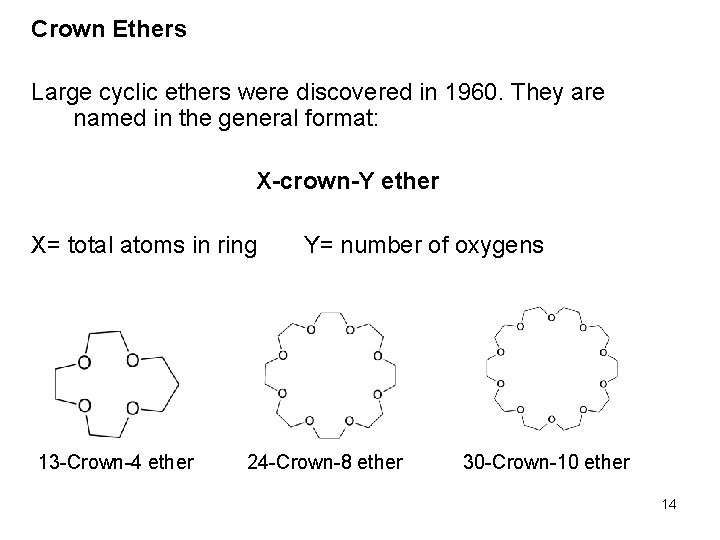
Crown Ethers Large cyclic ethers were discovered in 1960. They are named in the general format: X-crown-Y ether X= total atoms in ring 13 -Crown-4 ether Y= number of oxygens 24 -Crown-8 ether 30 -Crown-10 ether 14

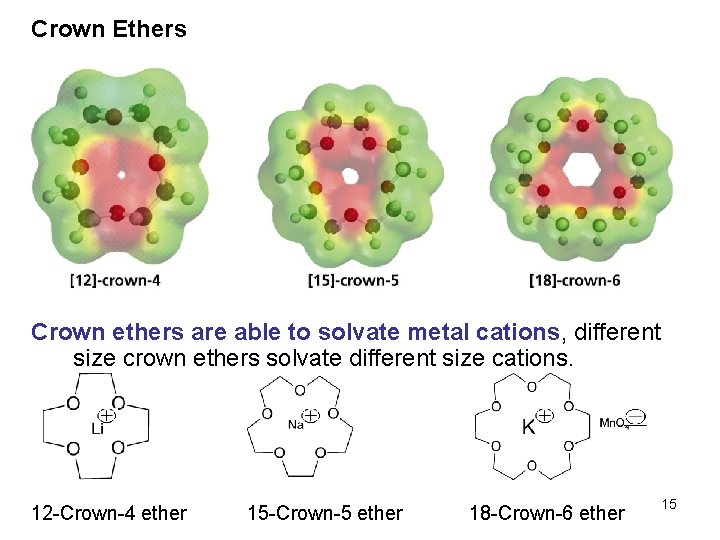
Crown Ethers Crown ethers are able to solvate metal cations, different size crown ethers solvate different size cations. 12 -Crown-4 ether 15 -Crown-5 ether 18 -Crown-6 ether 15

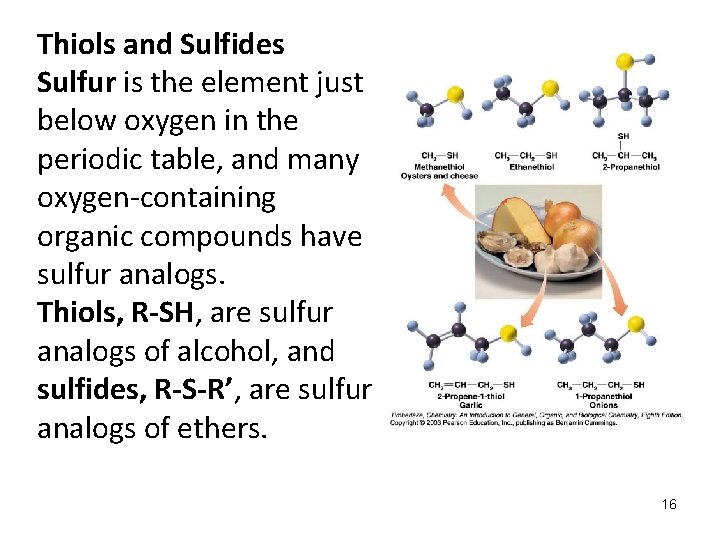
Thiols and Sulfides Sulfur is the element just below oxygen in the periodic table, and many oxygen-containing organic compounds have sulfur analogs. Thiols, R-SH, are sulfur analogs of alcohol, and sulfides, R-S-R’, are sulfur analogs of ethers. 16

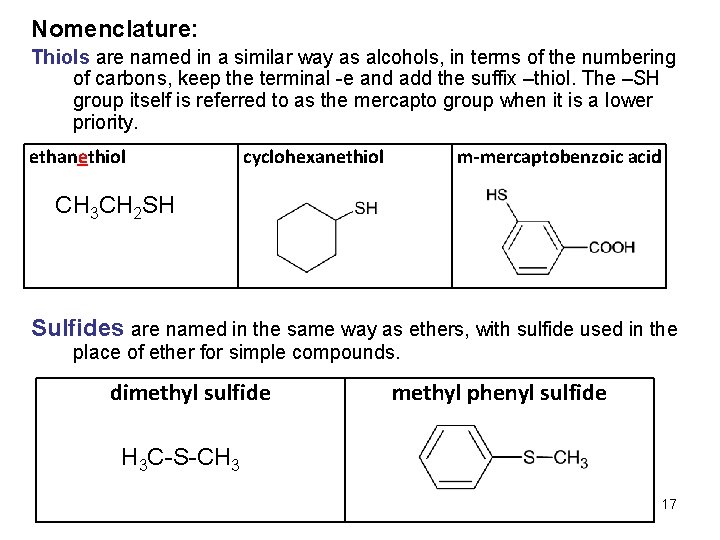
Nomenclature: Thiols are named in a similar way as alcohols, in terms of the numbering of carbons, keep the terminal -e and add the suffix –thiol. The –SH group itself is referred to as the mercapto group when it is a lower priority. ethanethiol cyclohexanethiol m-mercaptobenzoic acid CH 3 CH 2 SH Sulfides are named in the same way as ethers, with sulfide used in the place of ether for simple compounds. dimethyl sulfide methyl phenyl sulfide H 3 C-S-CH 3 17

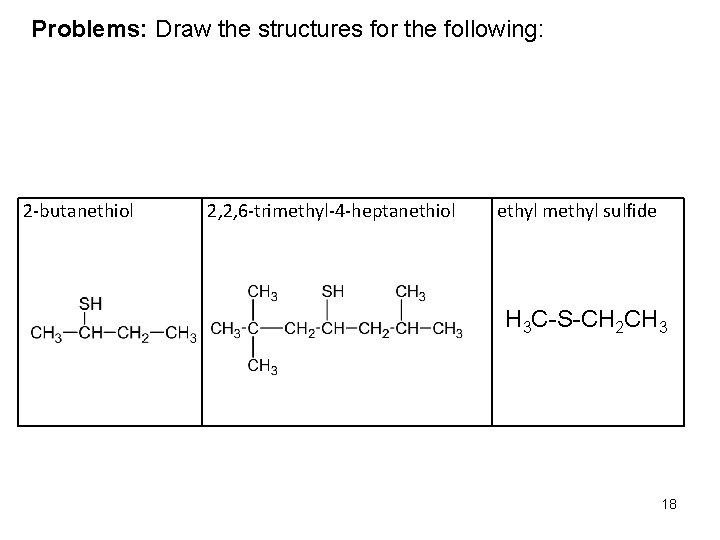
Problems: Draw the structures for the following: 2 -butanethiol 2, 2, 6 -trimethyl-4 -heptanethiol ethyl methyl sulfide H 3 C-S-CH 2 CH 3 18

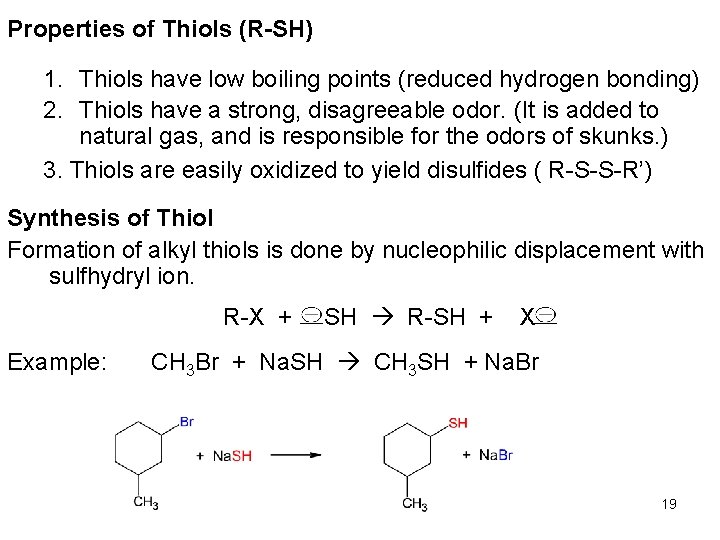
Properties of Thiols (R-SH) 1. Thiols have low boiling points (reduced hydrogen bonding) 2. Thiols have a strong, disagreeable odor. (It is added to natural gas, and is responsible for the odors of skunks. ) 3. Thiols are easily oxidized to yield disulfides ( R-S-S-R’) Synthesis of Thiol Formation of alkyl thiols is done by nucleophilic displacement with sulfhydryl ion. R-X + Example: -SH R-SH + X- CH 3 Br + Na. SH CH 3 SH + Na. Br 19

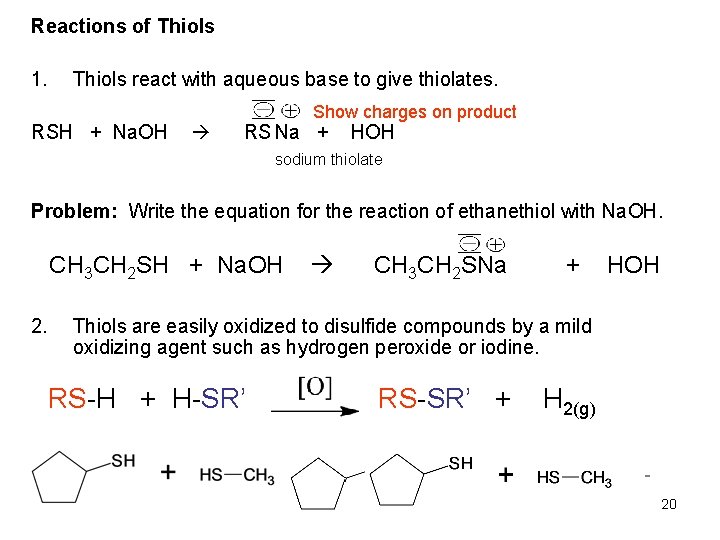
Reactions of Thiols 1. Thiols react with aqueous base to give thiolates. RSH + Na. OH Show charges on product RS Na + HOH sodium thiolate Problem: Write the equation for the reaction of ethanethiol with Na. OH. CH 3 CH 2 SH + Na. OH 2. CH 3 CH 2 SNa + HOH Thiols are easily oxidized to disulfide compounds by a mild oxidizing agent such as hydrogen peroxide or iodine. RS-H + H-SR’ RS-SR’ + H 2(g) 20

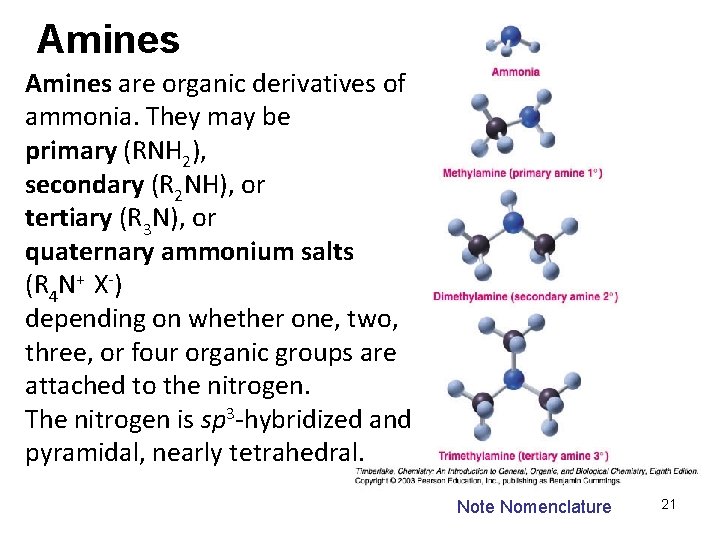
Amines are organic derivatives of ammonia. They may be primary (RNH 2), secondary (R 2 NH), or tertiary (R 3 N), or quaternary ammonium salts (R 4 N+ X-) depending on whether one, two, three, or four organic groups are attached to the nitrogen. The nitrogen is sp 3 -hybridized and pyramidal, nearly tetrahedral. Note Nomenclature 21

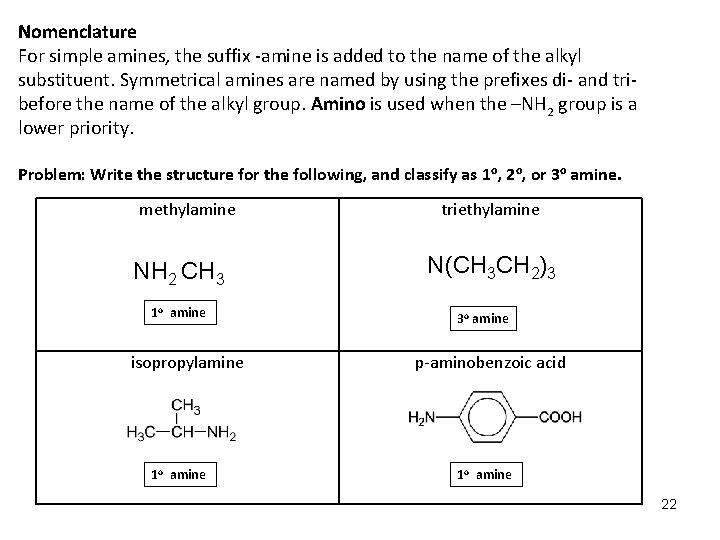
Nomenclature For simple amines, the suffix -amine is added to the name of the alkyl substituent. Symmetrical amines are named by using the prefixes di- and tribefore the name of the alkyl group. Amino is used when the –NH 2 group is a lower priority. Problem: Write the structure for the following, and classify as 1 o, 2 o, or 3 o amine. methylamine NH 2 CH 3 1 o amine isopropylamine 1 o amine triethylamine N(CH 3 CH 2)3 3 o amine p-aminobenzoic acid 1 o amine 22

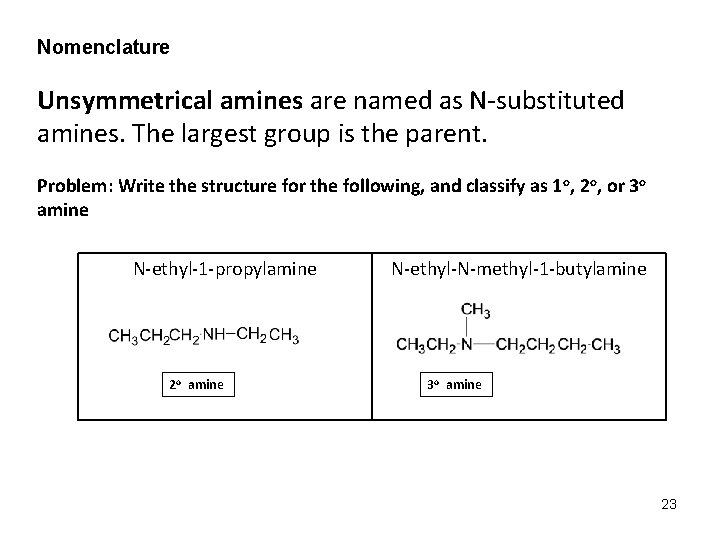
Nomenclature Unsymmetrical amines are named as N-substituted amines. The largest group is the parent. Problem: Write the structure for the following, and classify as 1 o, 2 o, or 3 o amine N-ethyl-1 -propylamine 2 o amine N-ethyl-N-methyl-1 -butylamine 3 o amine 23

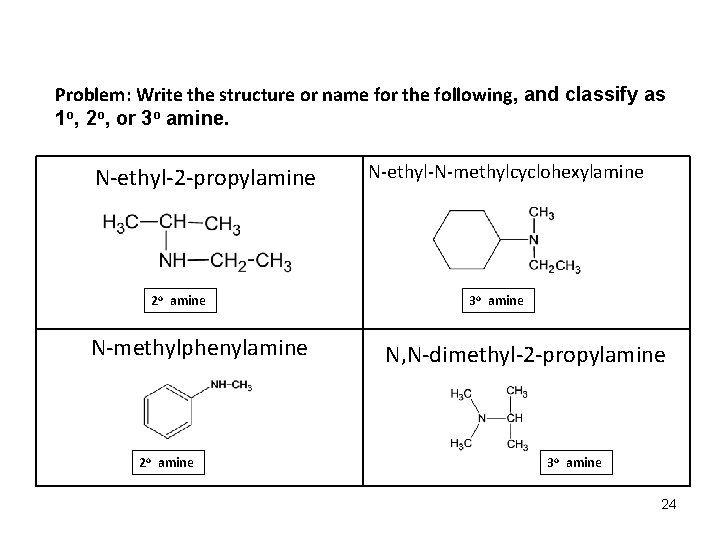
Problem: Write the structure or name for the following, and classify as 1 o, 2 o, or 3 o amine. N-ethyl-2 -propylamine 2 o amine N-methylphenylamine 2 o amine N-ethyl-N-methylcyclohexylamine 3 o amine N, N-dimethyl-2 -propylamine 3 o amine 24

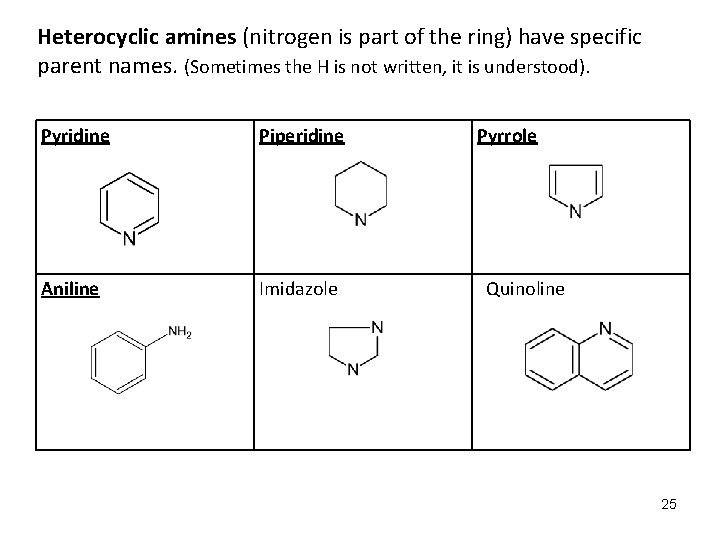
Heterocyclic amines (nitrogen is part of the ring) have specific parent names. (Sometimes the H is not written, it is understood). Pyridine Piperidine Aniline Imidazole Pyrrole Quinoline 25

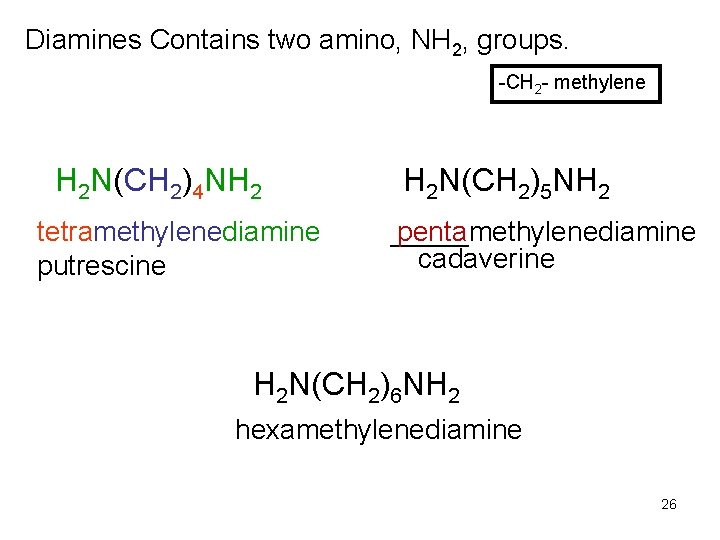
Diamines Contains two amino, NH 2, groups. -CH 2 - methylene H 2 N(CH 2)4 NH 2 tetramethylenediamine putrescine H 2 N(CH 2)5 NH 2 penta _____methylenediamine cadaverine H 2 N(CH 2)6 NH 2 hexamethylenediamine 26

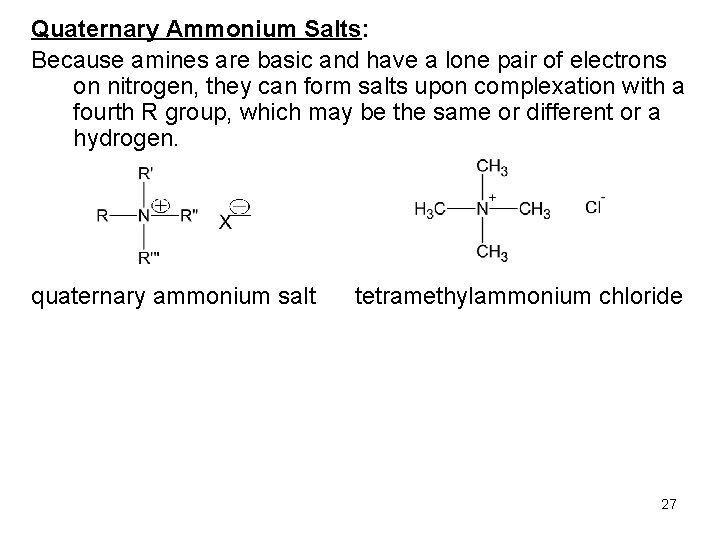
Quaternary Ammonium Salts: Because amines are basic and have a lone pair of electrons on nitrogen, they can form salts upon complexation with a fourth R group, which may be the same or different or a hydrogen. quaternary ammonium salt tetramethylammonium chloride 27

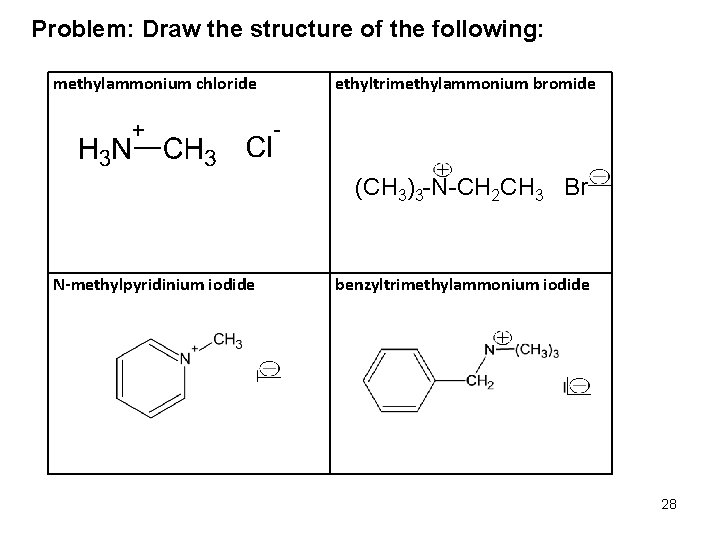
Problem: Draw the structure of the following: methylammonium chloride ethyltrimethylammonium bromide (CH 3)3 -N-CH 2 CH 3 Br- N-methylpyridinium iodide benzyltrimethylammonium iodide 28

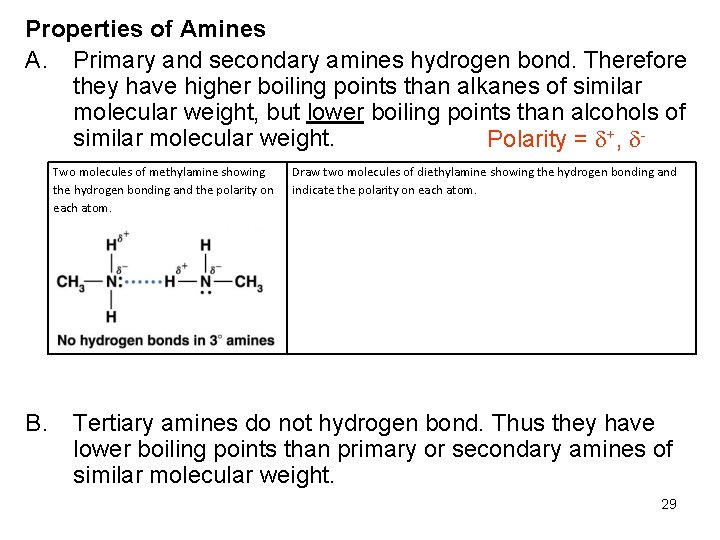
Properties of Amines A. Primary and secondary amines hydrogen bond. Therefore they have higher boiling points than alkanes of similar molecular weight, but lower boiling points than alcohols of similar molecular weight. Polarity = +, Two molecules of methylamine showing the hydrogen bonding and the polarity on each atom. B. Draw two molecules of diethylamine showing the hydrogen bonding and indicate the polarity on each atom. Tertiary amines do not hydrogen bond. Thus they have lower boiling points than primary or secondary amines of similar molecular weight. 29

C. Low molecular weight amines are gases, higher molecular weight amines are liquids. D. Low molecular weight amines are volatile and have unpleasant ammonia like or fishy odors. E. Low molecular weight amines are water soluble. F. Many amines are physiologically important and some are toxic. G. Amines are weak Br nsted-Lowery Bases. 30
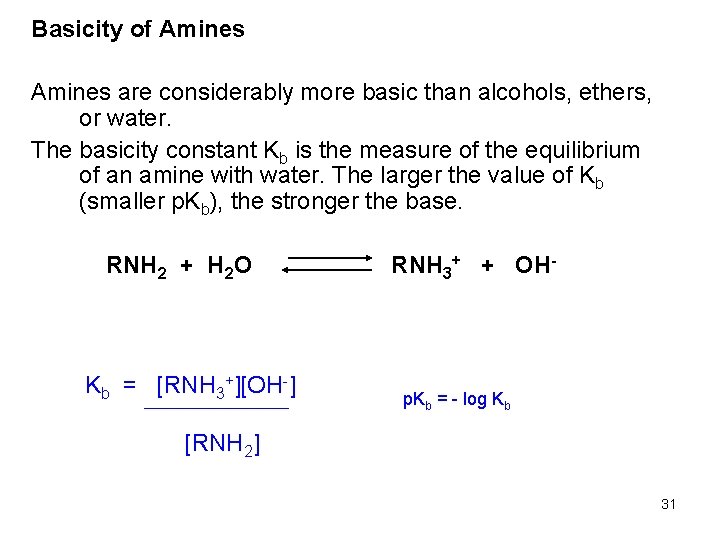
Basicity of Amines are considerably more basic than alcohols, ethers, or water. The basicity constant Kb is the measure of the equilibrium of an amine with water. The larger the value of Kb (smaller p. Kb), the stronger the base. RNH 2 + H 2 O Kb = [RNH 3+][OH-] RNH 3+ + OH- p. Kb = - log Kb [RNH 2] 31

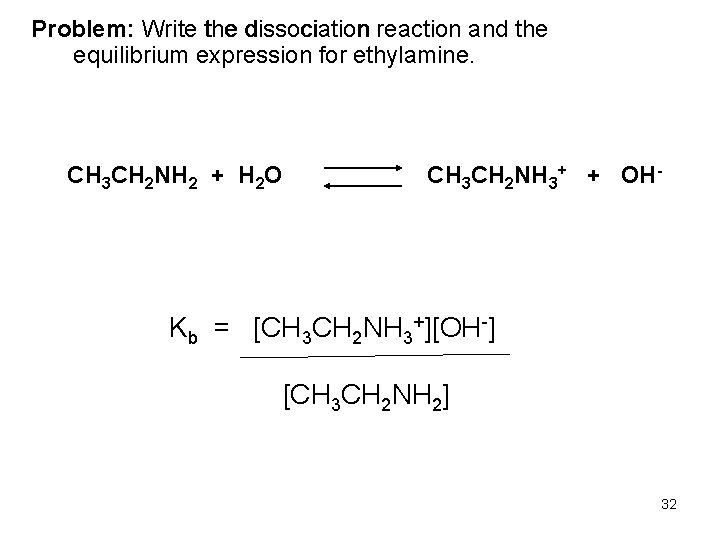
Problem: Write the dissociation reaction and the equilibrium expression for ethylamine. CH 3 CH 2 NH 2 + H 2 O CH 3 CH 2 NH 3+ + OH- Kb = [CH 3 CH 2 NH 3+][OH-] [CH 3 CH 2 NH 2] 32

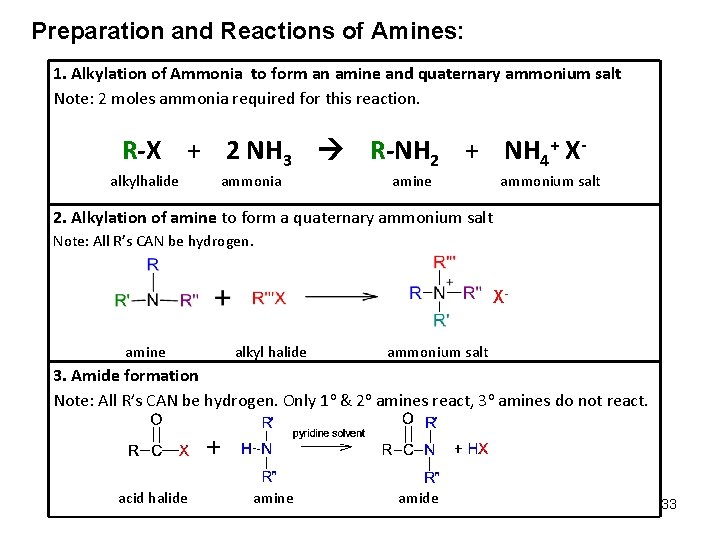
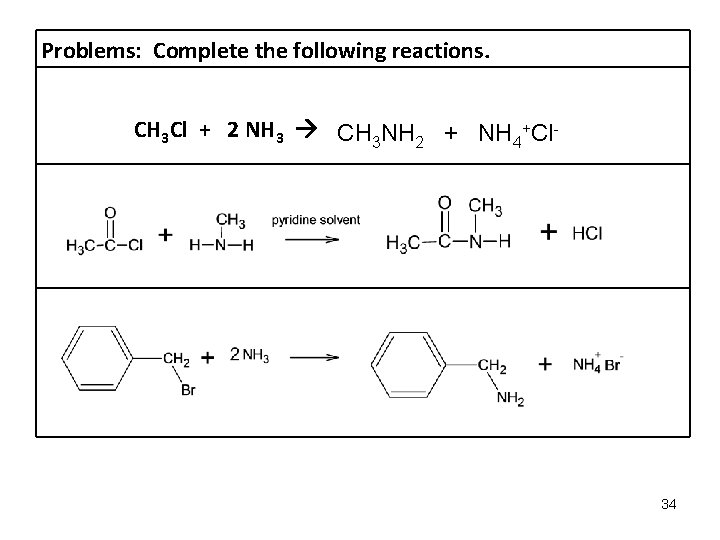
Preparation and Reactions of Amines: 1. Alkylation of Ammonia to form an amine and quaternary ammonium salt Note: 2 moles ammonia required for this reaction. R-X + 2 NH 3 R-NH 2 + NH 4+ Xalkylhalide ammonia amine ammonium salt 2. Alkylation of amine to form a quaternary ammonium salt Note: All R’s CAN be hydrogen. Xamine alkyl halide ammonium salt 3. Amide formation Note: All R’s CAN be hydrogen. Only 1 o & 2 o amines react, 3 o amines do not react. acid halide amine amide 33

Problems: Complete the following reactions. CH 3 Cl + 2 NH 3 CH 3 NH 2 + NH 4+Cl- 34

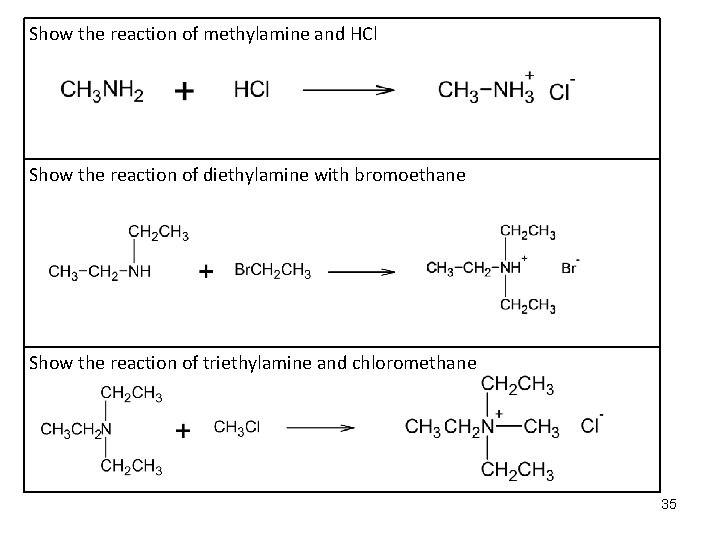
Show the reaction of methylamine and HCl Show the reaction of diethylamine with bromoethane Show the reaction of triethylamine and chloromethane 35

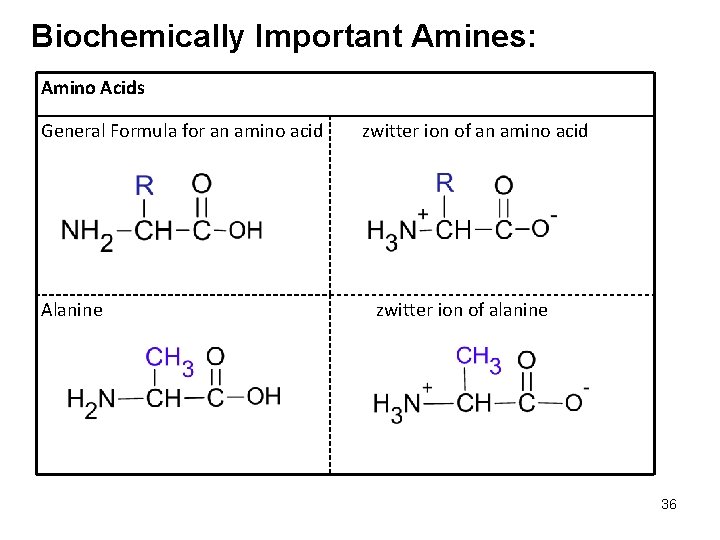
Biochemically Important Amines: Amino Acids General Formula for an amino acid Alanine zwitter ion of an amino acid zwitter ion of alanine 36

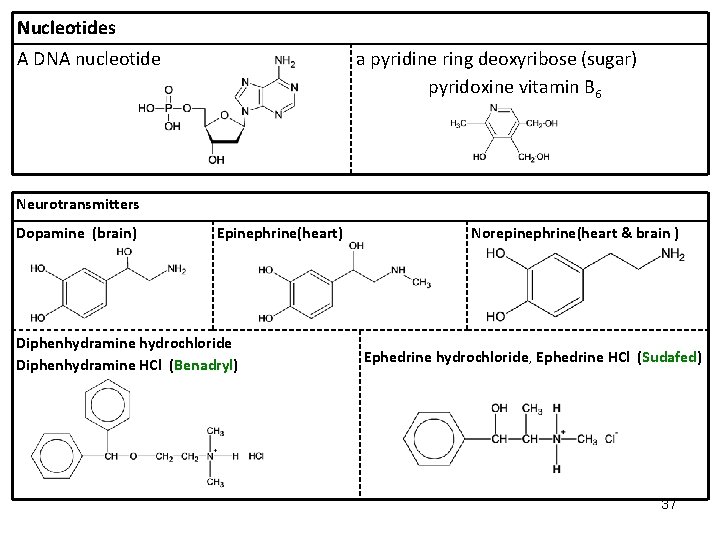
Nucleotides A DNA nucleotide a pyridine ring deoxyribose (sugar) pyridoxine vitamin B 6 Neurotransmitters Dopamine (brain) Epinephrine(heart) Diphenhydramine hydrochloride Diphenhydramine HCl (Benadryl) Norepinephrine(heart & brain ) Ephedrine hydrochloride, Ephedrine HCl (Sudafed) 37

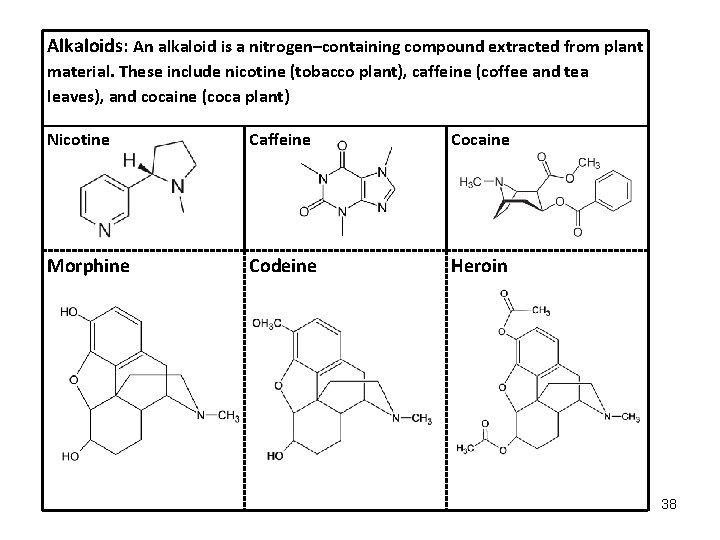
Alkaloids: An alkaloid is a nitrogen–containing compound extracted from plant material. These include nicotine (tobacco plant), caffeine (coffee and tea leaves), and cocaine (coca plant) Nicotine Caffeine Cocaine Morphine Codeine Heroin 38