SECTION 3 COMPOUND NAMES AND FORMULAS KEY IDEAS










- Slides: 13

SECTION 3: COMPOUND NAMES AND FORMULAS

KEY IDEAS � How are ionic compounds named? � What do the numerical prefixes used in naming covalent compounds tell you? � What does a compound´s empirical formula indicate?

KEY TERMS Empirical formula Anion Molecular formula Cation

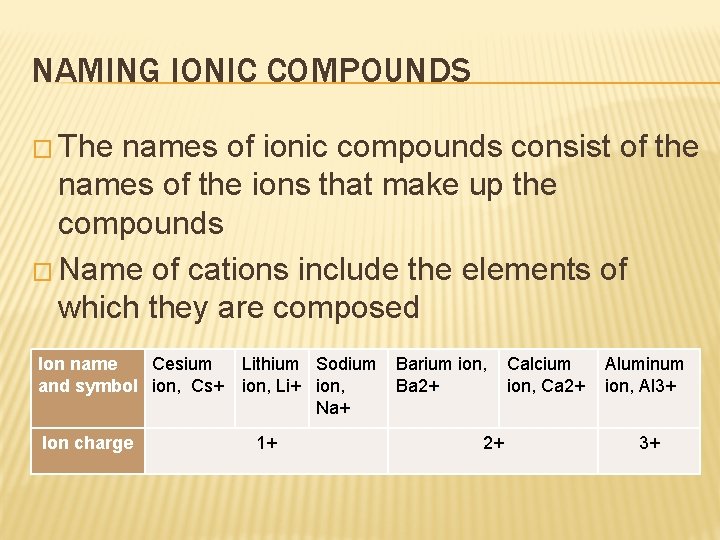
NAMING IONIC COMPOUNDS � The names of ionic compounds consist of the names of the ions that make up the compounds � Name of cations include the elements of which they are composed Ion name Cesium and symbol ion, Cs+ Ion charge Lithium Sodium ion, Li+ ion, Na+ 1+ Barium ion, Ba 2+ 2+ Calcium ion, Ca 2+ Aluminum ion, Al 3+ 3+

NAMING IONIC COMPOUNDS � Names of anions are altered names of elements � Na. F: is made of sodium ions, Na+, and fluoride ions, F-. Therefore, its name is sodium fluoride � An ionic compound must have a total charge of zero � If an ionic compound is made up of ions that have different charges, the ratio will not be 1: 1 � For calcium fluoride to have a total charge of zero, there must be two fluoride ions for every calcium

NAMING IONIC COMPOUNDS � Some � The cation names must show their charge of the iron cation in Fe 2 O 3 is different from the charge of the iron cation in Fe. O. In cases such as this, the cation name must be followed by a Roman numeral in parentheses that shows the cation’s charge. � Fe 2 O 3 is made of Fe 3+ ions, so it is named iron (III) oxide. Fe. O is made of Fe 2+ ions, so it is named iron (II) oxide.

NAMING IONIC COMPOUNDS � Determine cation the charge of a transition metal � How can you tell that the iron in Fe 2 O 3 has a charge of 3+? � Write formulas for ionic compound using names � You can find the charge of each ion in a compound if you are given the compound’s formula. You can find the formula for a compound if you are given the compound’s name

NAMING IONIC COMPOUNDS � Math � Go Skills: Writing Ionic Formulas to go. hrw. com and enter keyword HK 8 MP

NAMING COVALENT COMPOUNDS � For covalent compounds or two elements, numerical prefixes tell how many atoms of each element are in the molecule � Numerical prefixes are used to name covalent compounds of two elements � If there is only one atom of the first element, the name does not get a prefix � The element farther to the right in the periodic table is named second and ends in –ide � One boron atom and three fluorine atoms make up boron trifluoride, BF 3

EMPIRICAL FORMULAS � An empirical formula tells us the smallest whole-number ratio of atoms that are in a compound � Different compounds can have same empirical formula � Molecular formulas are determined from empirical formulas � Masses can be used to determine empirical formulas

EMPIRICAL FORMULAS � Empirical formula: the composition of a compound in terms of the relative number and kinds of atoms in the simplest ratio � Molecular formula: a chemical formula that shows the number and kinds of atoms in a molecule, but not the arrengement of atoms

EMPIRICAL FORMULAS � Math � Go Skills: Finding Empirical Formulas to go. hrw. com and enter keyword HK 8 MP

HOMEWORK