Section 2 Respiratory Gases Exchange Partial pressure The












































- Slides: 75

Section 2 Respiratory Gases Exchange



• Partial pressure – The pressure exerted by each type of gas in a mixture – Partial pressure=total pressure x percent of volume • Diffusion of gases through liquids – Concentration of a gas in a liquid is determined by its partial pressure and its solubility.







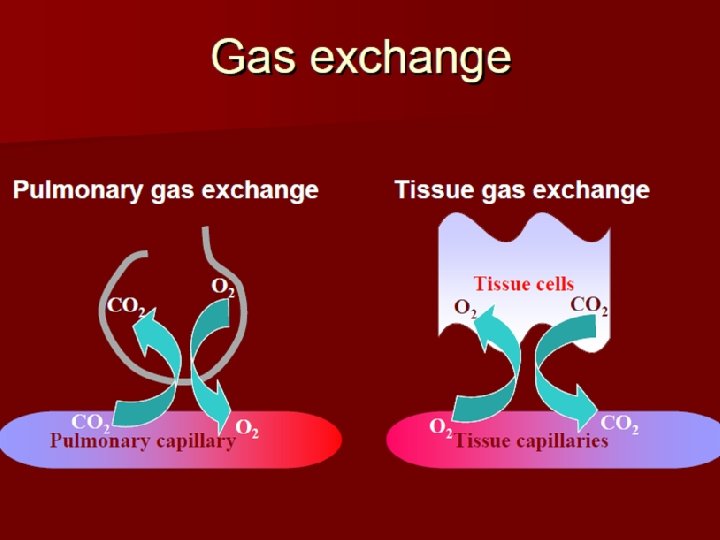
Oxygen and Carbon Dioxide Diffusion Gradients • Oxygen – Moves from alveoli into blood. Blood is almost completely saturated with oxygen when it leaves the capillary – Oxygen moves from tissue capillaries into the tissues • Carbon dioxide – Moves from tissues into tissue capillaries – Moves from pulmonary capillaries into the alveoli


Capillary Basement membrane Epithelial Basement membrane 0. 2 micrometer

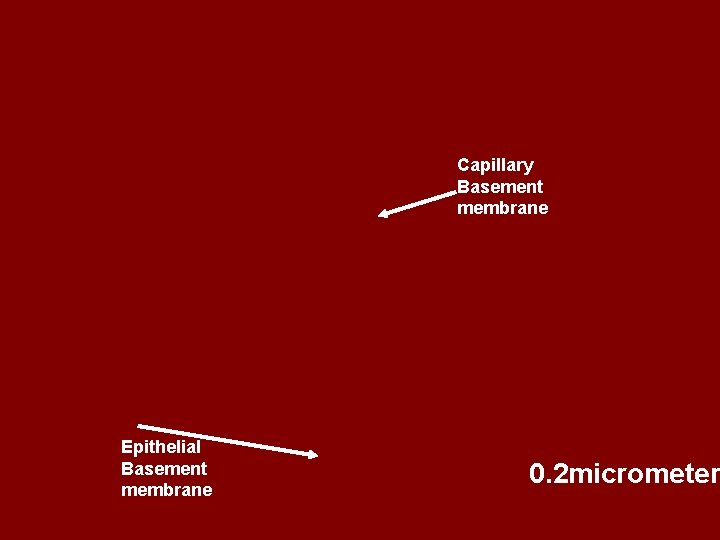
• Diffusion distance—thickness of alveolar membrane (inverse ratio relationship) • Pulmonary fibrosis---Thickness increases • Pulmonary edema---Diffusion decreases } •

• Diffusion area of alveolar membrane : when diffusion area of alveolar membrane is large, it diffuses fast. • diffusion area of alveolar membrane is 40 m 2 in normal quiet state. • diffusion area of alveolar membrane is 70 m 2 during sports. • diffusion area of alveolar membrane decreases in disease.

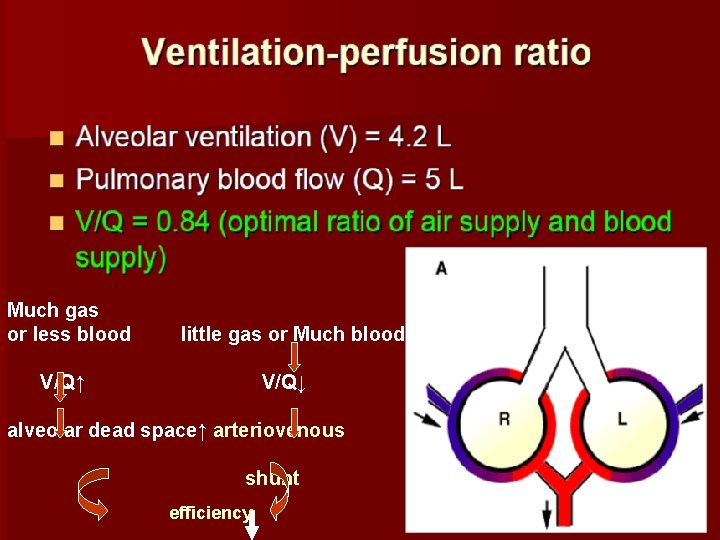
Much gas or less blood little gas or Much blood V/Q↑ V/Q↓ alveolar dead space↑ arteriovenous shunt efficiency

partial alveolar gas can not exchange fully with the blood e. g. pulmonary embolism ventilation /perfusion ratio decreases: it means partial blood flow through hypoventilation alveoli. They can not get fully exchange. And it equals functional arteriovenous shunt.

3. 3 Upright position 0. 63

Internal Respiration • All cells require oxygen for metabolism • All cells require means to remove carbon dioxide • Gas exchange at cellular level

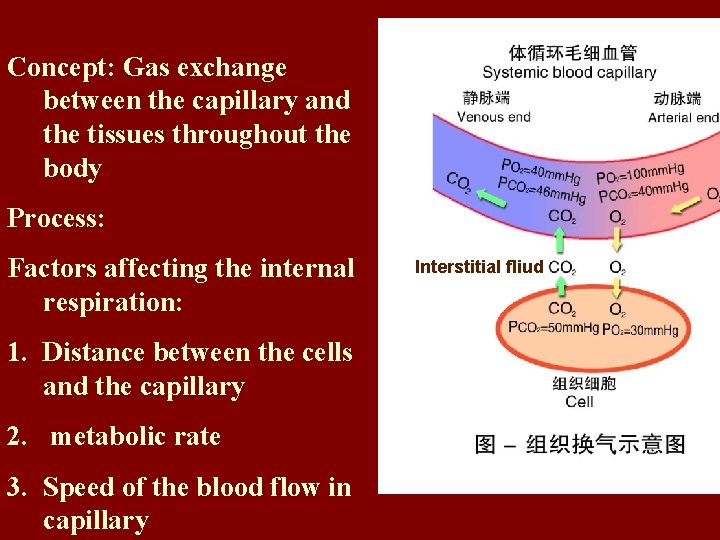
Concept: Gas exchange between the capillary and the tissues throughout the body Process: Factors affecting the internal respiration: 1. Distance between the cells and the capillary 2. metabolic rate 3. Speed of the blood flow in capillary Interstitial fliud

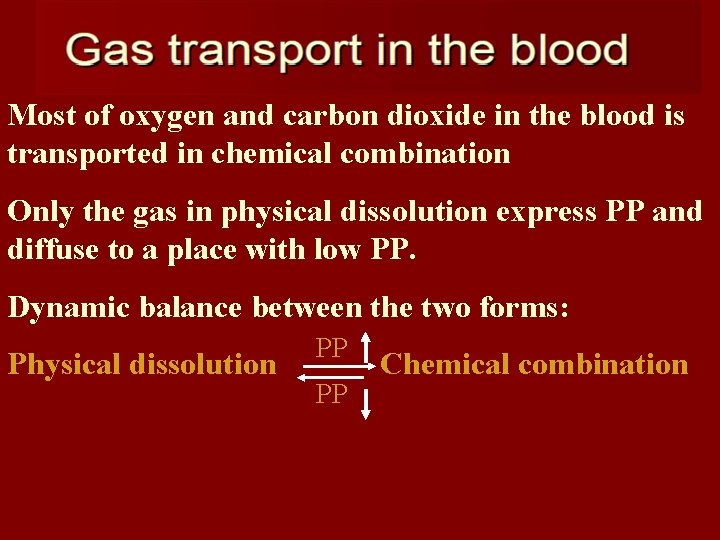
Most of oxygen and carbon dioxide in the blood is transported in chemical combination Only the gas in physical dissolution express PP and diffuse to a place with low PP. Dynamic balance between the two forms: Physical dissolution PP PP Chemical combination

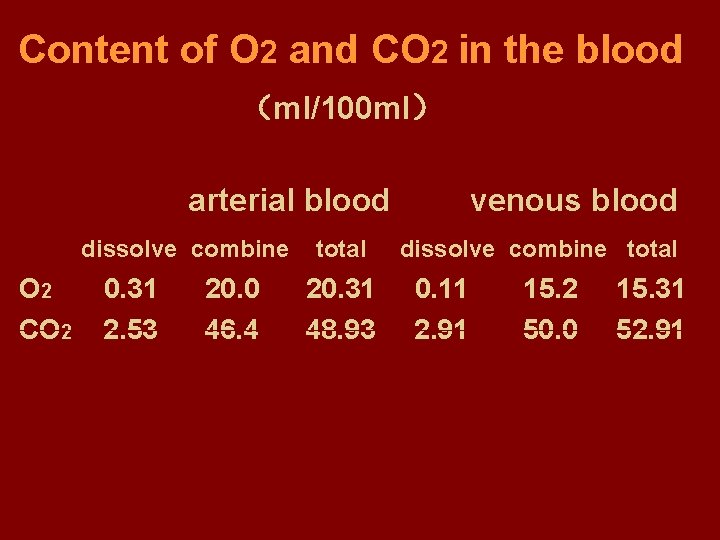
Content of O 2 and CO 2 in the blood (ml/100 ml) arterial blood dissolve combine O 2 CO 2 0. 31 2. 53 20. 0 46. 4 total 20. 31 48. 93 venous blood dissolve combine total 0. 11 2. 91 15. 2 50. 0 15. 31 52. 91



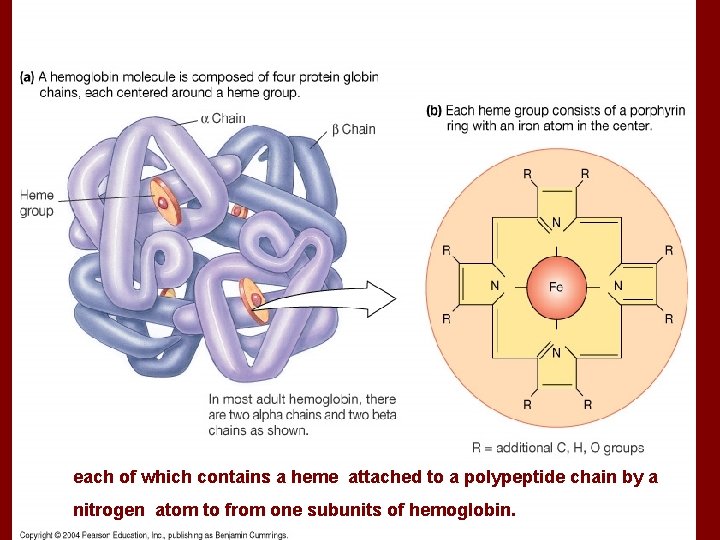
each of which contains a heme attached to a polypeptide chain by a nitrogen atom to from one subunits of hemoglobin.


Oxygen

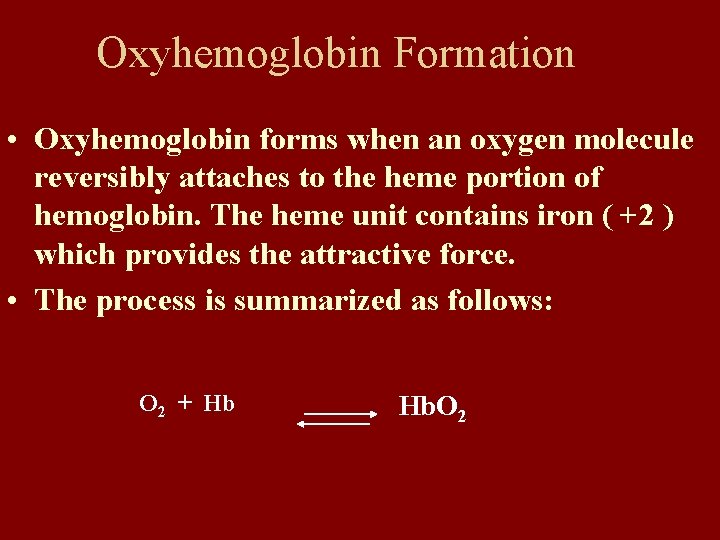
Oxyhemoglobin Formation • Oxyhemoglobin forms when an oxygen molecule reversibly attaches to the heme portion of hemoglobin. The heme unit contains iron ( +2 ) which provides the attractive force. • The process is summarized as follows: O 2 + Hb Hb. O 2

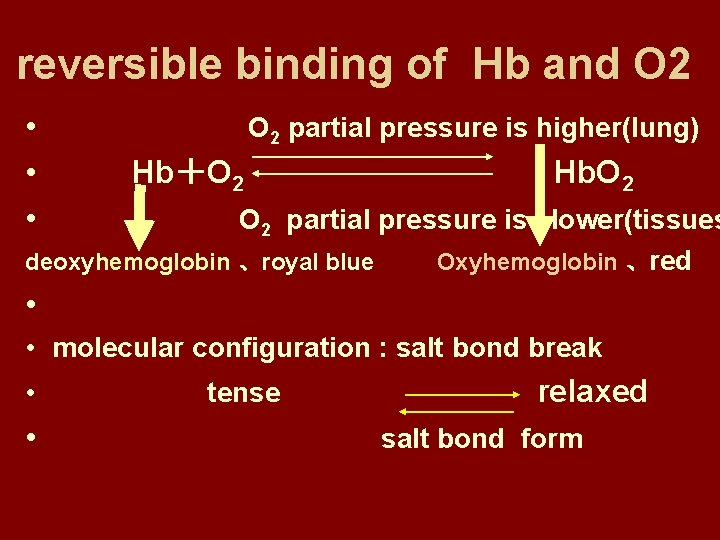
character • 1. Reversible binding. Without enzyme. Fast. Effected by PO 2. • In lungs, increasing of PO 2 promotes combination. • In tissues, decreasing of PO 2 promotes releasing.


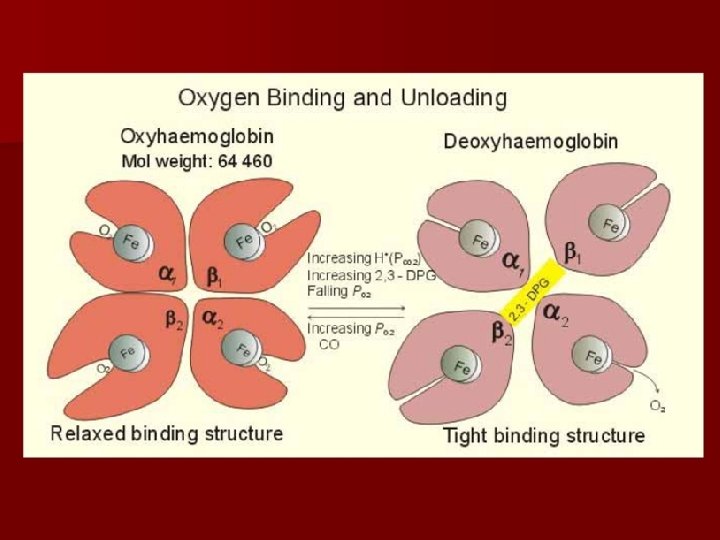
reversible binding of Hb and O 2 • • • O 2 partial pressure is higher(lung) Hb+O 2 Hb. O 2 partial pressure is lower(tissues deoxyhemoglobin 、royal blue Oxyhemoglobin 、red • • molecular configuration : salt bond break • • tense relaxed salt bond form

Hb binds with O 2 —salt bond breaks, R form,the affinity of R form to O 2 is larger. Hb dissociates with O 2—salt bond forms, T form,the affinity of T form to O 2 is smaller.


character • 2. O 2 binds with Fe 2+ of ferrohemoglobin. The ion value is permanent. The iron stays in the ferrous state, So the process is called oxygenation but not oxidation.

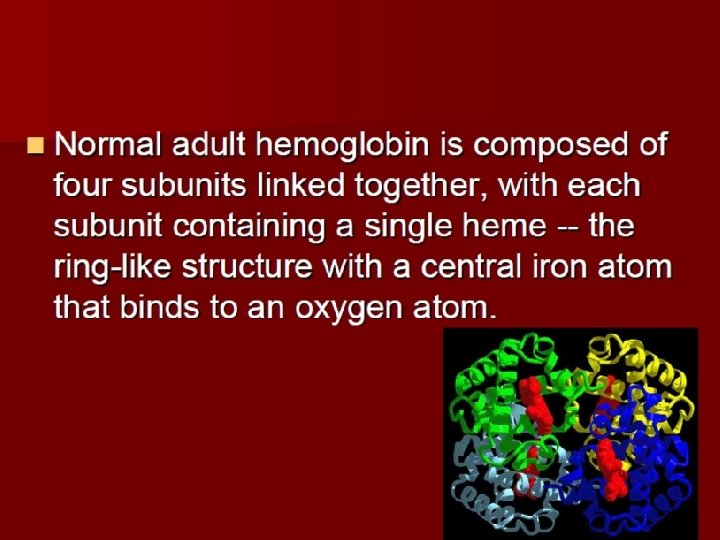
• 3. Globin of ferrohemoglobin is made up of two αpeptide chains and two β peptide chains. There is a heme molecular on each peptide chain including a Fe 2+. Each Fe 2+ binds with an O 2. • So each ferrohemoglobin can bind with four O 2. (Hb. O 8) • 1 g. Hb can bind with 1. 34-1. 39 ml. O 2.

In 15 g/100 ml blood , 1 g Hb binds with 1. 34 ml O 2. Oxygen capacity= 15× 1. 34= 20 ml arterial blood: 20 ml O 2 venous blood: 15 ml O 2

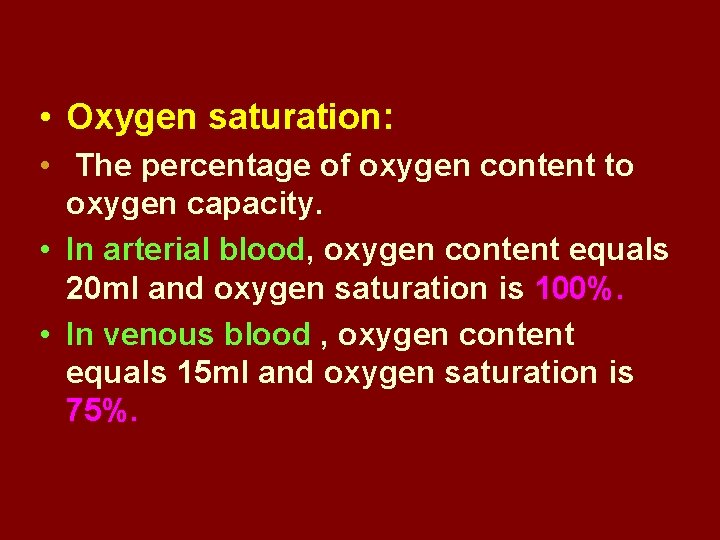
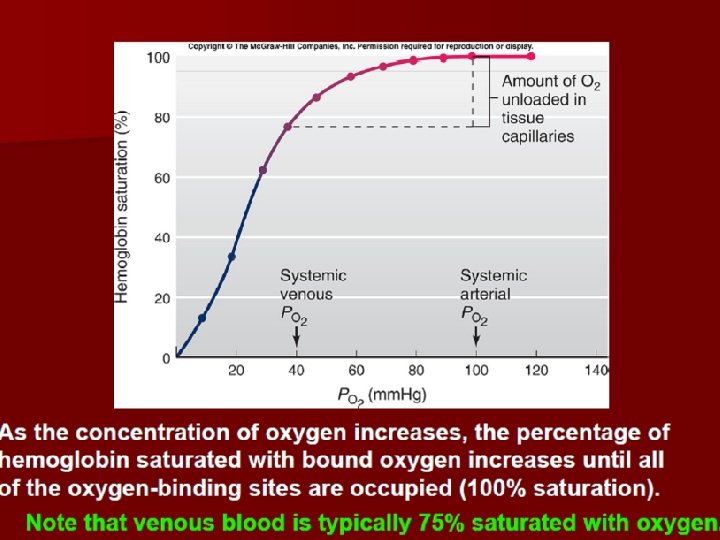
• Oxygen saturation: • The percentage of oxygen content to oxygen capacity. • In arterial blood, oxygen content equals 20 ml and oxygen saturation is 100%. • In venous blood , oxygen content equals 15 ml and oxygen saturation is 75%.



4. The binding or dissociation curves of Hb and O 2 appear S form. This is related to the allosterism effect of Hb.


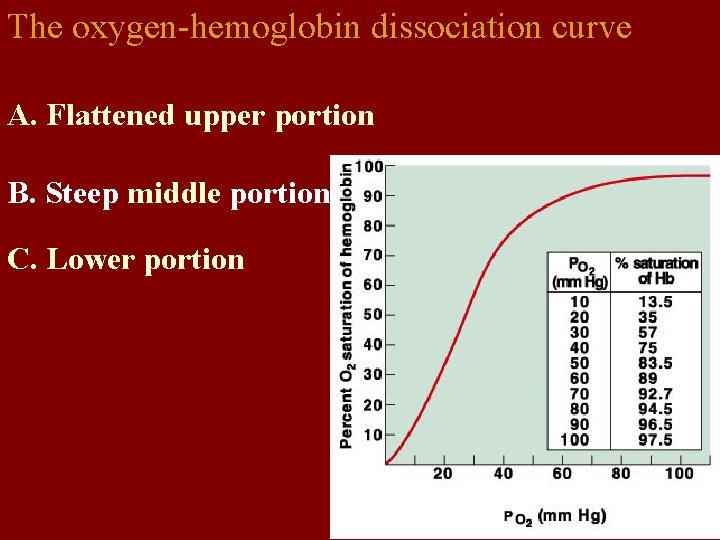
The oxygen-hemoglobin dissociation curve A. Flattened upper portion B. Steep middle portion C. Lower portion

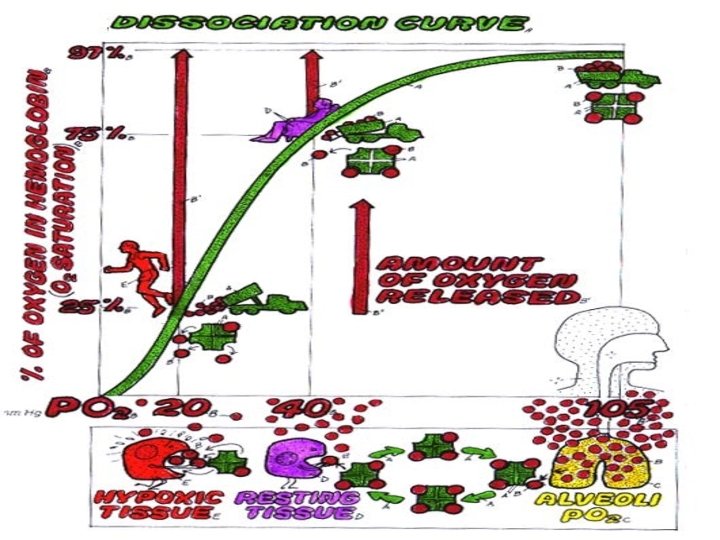
Binding zone • • Slope is flat. 1) Partial pressure of oxygen is high, oxygenation saturation is also high. 2) Partial pressure of oxygen changes greatly. But saturation changes little— even PO 2 of environment or alveoli descents, oxygenation saturation will maintain high level. When PO 2> 100 mm. Hg, rising of oxygenation saturation is not obvious. Rising of blood oxygen volume is little.


• 2. Middle segment of curve: • PO 2 60-40 mm. Hg. Hb. O 2 releases O 2. • At this time Hb oxygen saturation is 75%, oxygen content in blood is 14. 4 m. LO 2. • In the other words, every 100 ml blood releases 5 ml. O 2 when it flows over tissues. • The percentage of oxygen capacity released when blood flows over tissues to oxygen content in arteria is called oxygen utilization coefficient. It is 25% in normal quiet state and it increases to 75% in sports.

Storage of O 2 • The slope is steep. • PO 2 descents a little. It makes oxygen dissociation saturation descent. This is benefit to supplying oxygen for tissue activity. Oxygen utilization coefficient increases to 75%. •




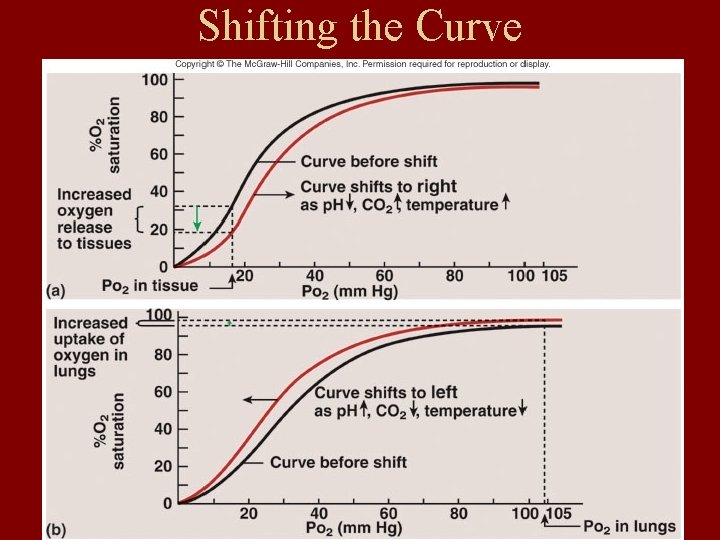
Shifting the Curve

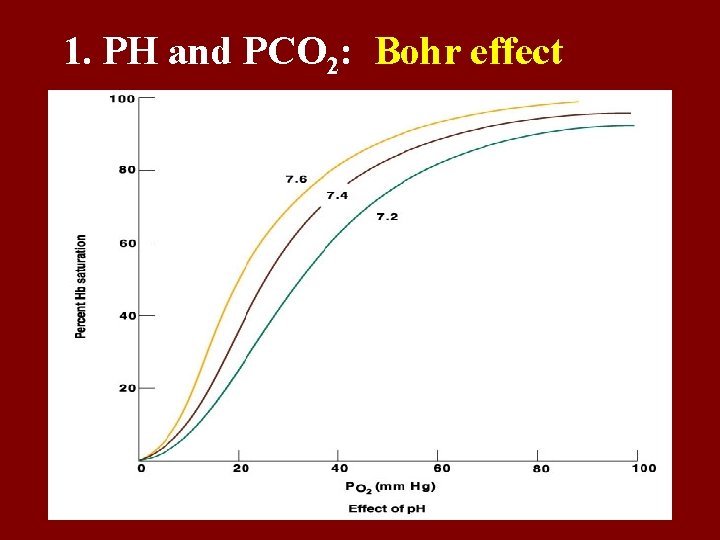
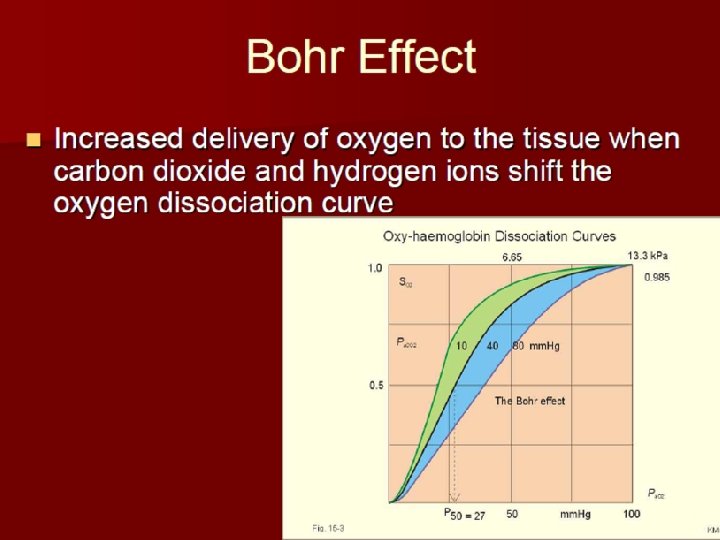
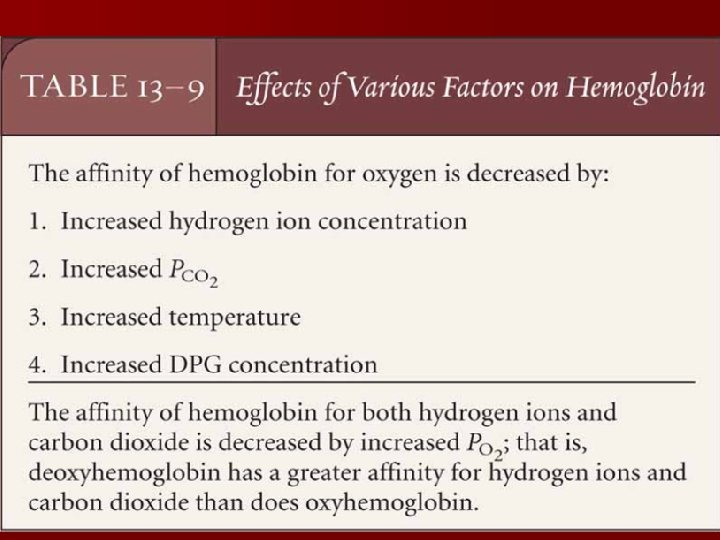
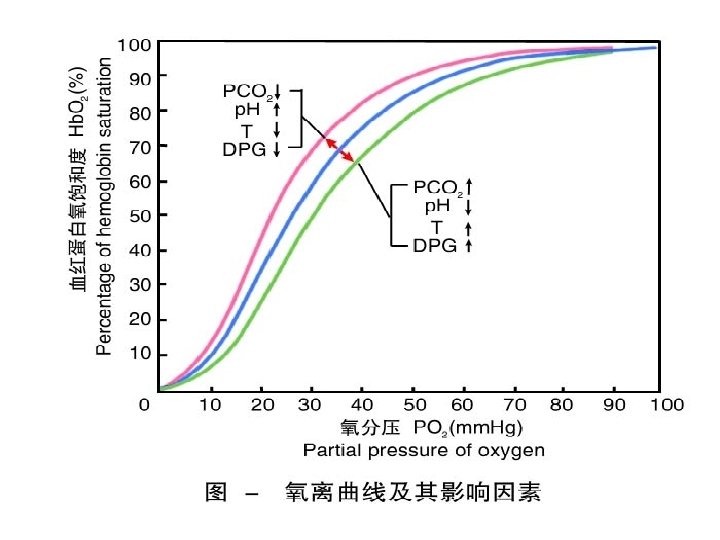
1. PH and PCO 2: Bohr effect



• Bohr effect : • PH ↓ and PCO 2 ↑—make salt bond form → T form→ Hb dissociates with O→ 2 Curve move to right. • Meaning: can promote oxygenation of lung capillary blood. And for dissociation of O 2 in tissue capillary blood.

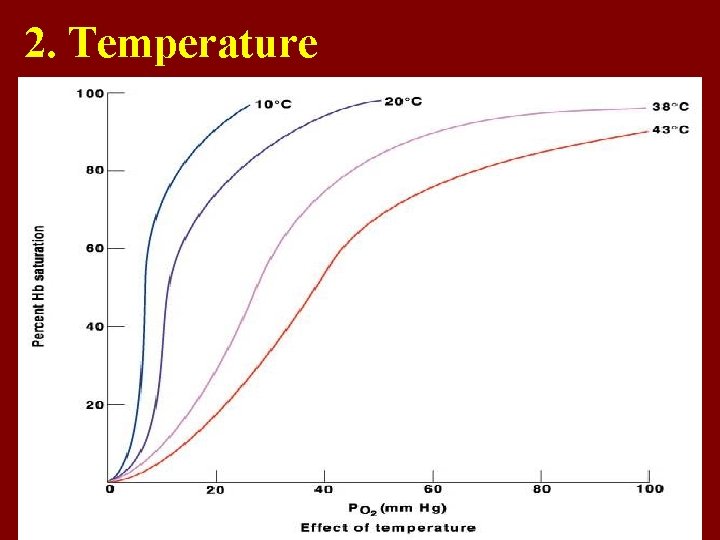
2. Temperature

• 2. effect of temperature • T ↑—affinity of Hb to O 2 ↓. Curve moves to ? right Activity of H+ increases.


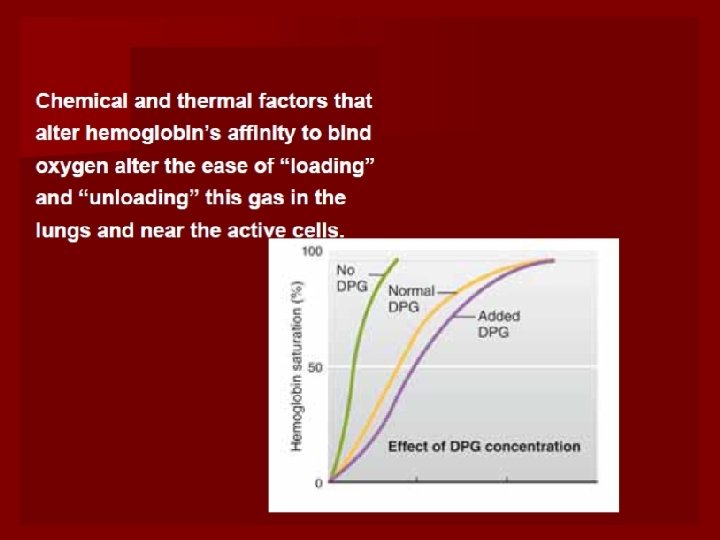
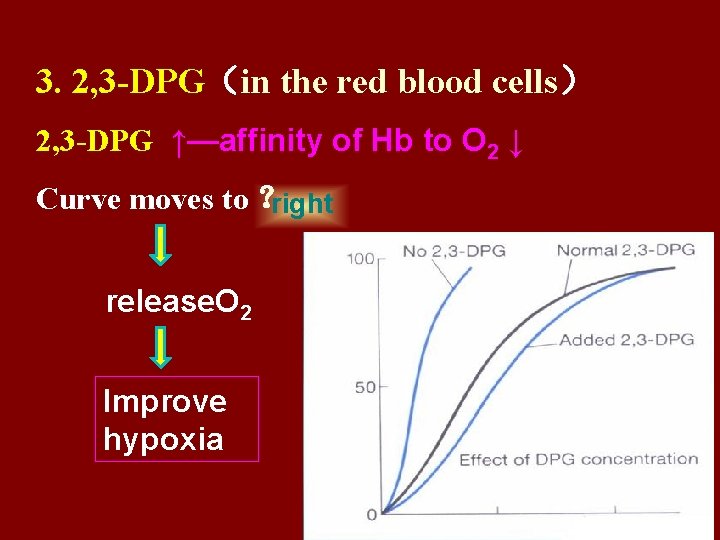
3. 2, 3 -DPG(in the red blood cells) 2, 3 -DPG ↑—affinity of Hb to O 2 ↓ Curve moves to ? right release. O 2 Improve hypoxia





Carbon Dioxide Transport Method • Dissolved in Plasma Percentage 5% • Chemically Bind To Hemoglobin 7% Bicarbonate Ion 88%

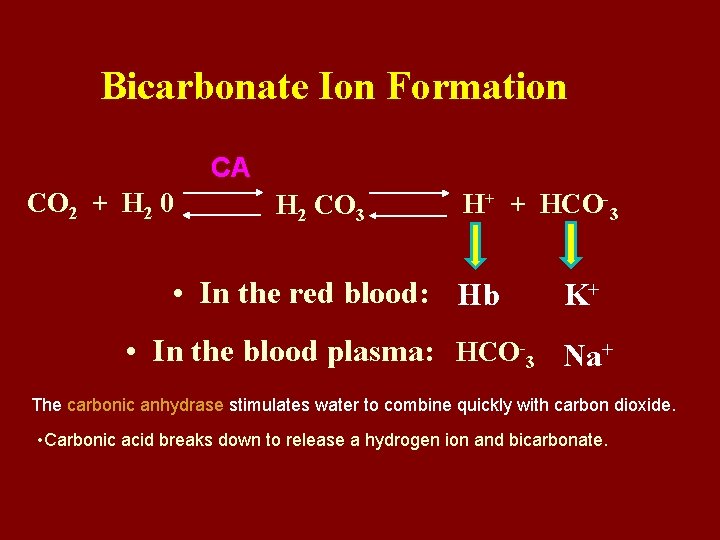
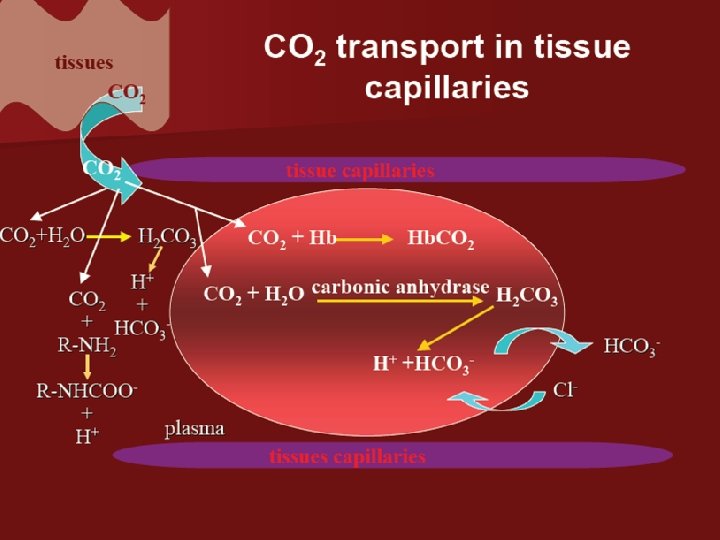
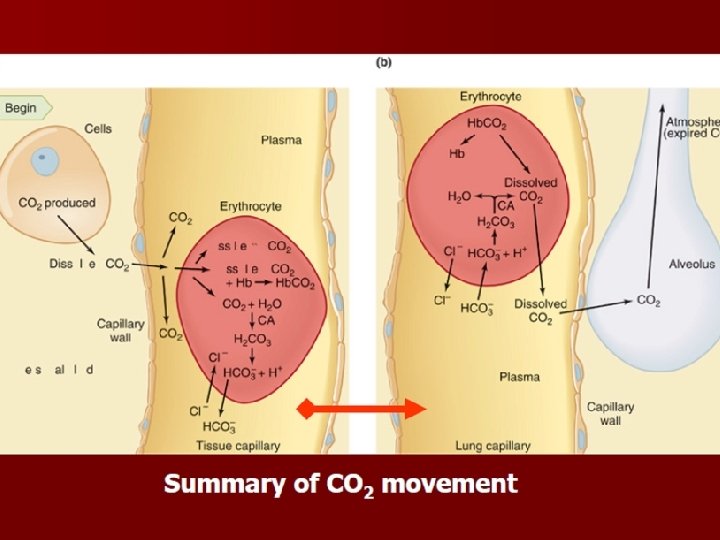
Bicarbonate Ion Formation CA CO 2 + H 2 0 H 2 CO 3 H+ + HCO-3 • In the red blood: Hb K+ • In the blood plasma: HCO-3 Na+ The carbonic anhydrase stimulates water to combine quickly with carbon dioxide. • Carbonic acid breaks down to release a hydrogen ion and bicarbonate.

character: • 1. Reaction is reversible. But it need the help of enzyme carbonic anhydrase. • 2. Conjugation or dissociation is decided by partial pressure of CO 2. • 3. There is the transfer of Cl- during the reaction.

Carbaminohemoglobin Formation • Carbon dioxide molecule reversibly attaches to an amino portion of hemoglobin. In tissue Hb. NH 2 O 2+H++CO 2 Hb. NHCOOH+O 2 In lungs

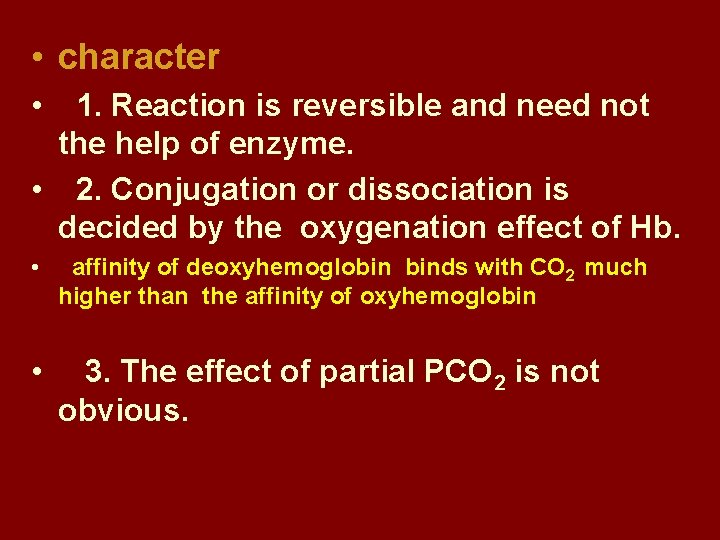
• character • 1. Reaction is reversible and need not the help of enzyme. • 2. Conjugation or dissociation is decided by the oxygenation effect of Hb. • affinity of deoxyhemoglobin binds with CO 2 much higher than the affinity of oxyhemoglobin • 3. The effect of partial PCO 2 is not obvious.




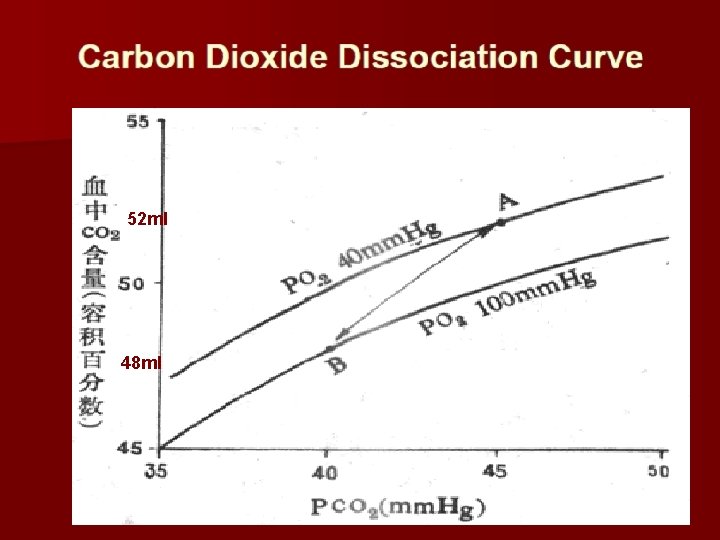
52 ml 48 ml

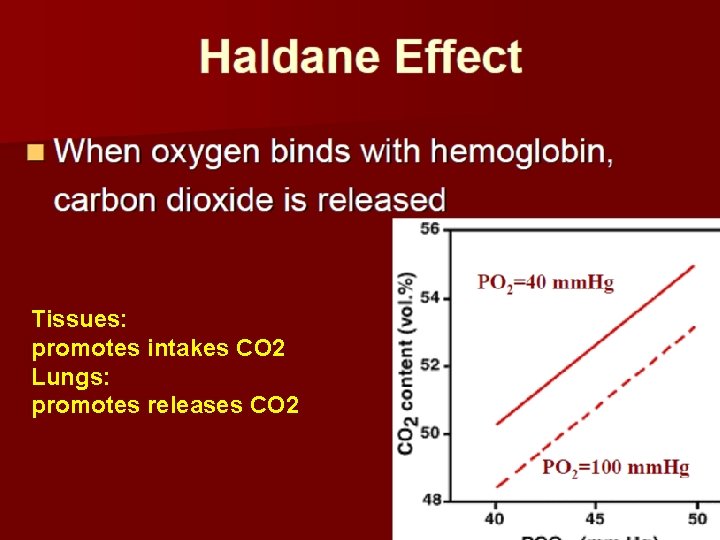
Tissues: promotes intakes CO 2 Lungs: promotes releases CO 2

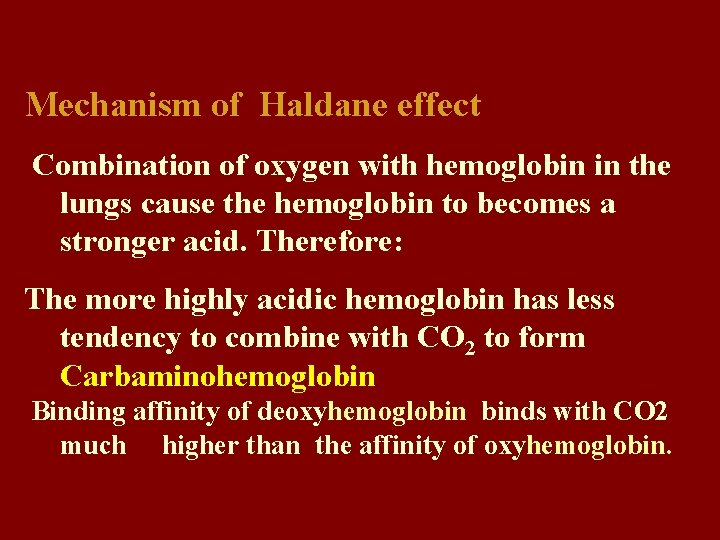
Mechanism of Haldane effect Combination of oxygen with hemoglobin in the lungs cause the hemoglobin to becomes a stronger acid. Therefore: The more highly acidic hemoglobin has less tendency to combine with CO 2 to form Carbaminohemoglobin Binding affinity of deoxyhemoglobin binds with CO 2 much higher than the affinity of oxyhemoglobin.


lung?