Section 2 Exploring the Periodic Table Learning Targets

Section 2 Exploring the Periodic Table

Learning Targets I can explain why atoms form ions. l I can determine the ion formation of an element. (worksheet) l I can differentiate between nonmetals, metals and metalloids. l
The Role of Electrons The periodic trends in the periodic table are the result of electron arrangement l Chemical properties are determined by valence electrons , which are located in the outermost energy level. l Elements in a group have chemical and physical properties in common because they have the same number of valence electrons. l
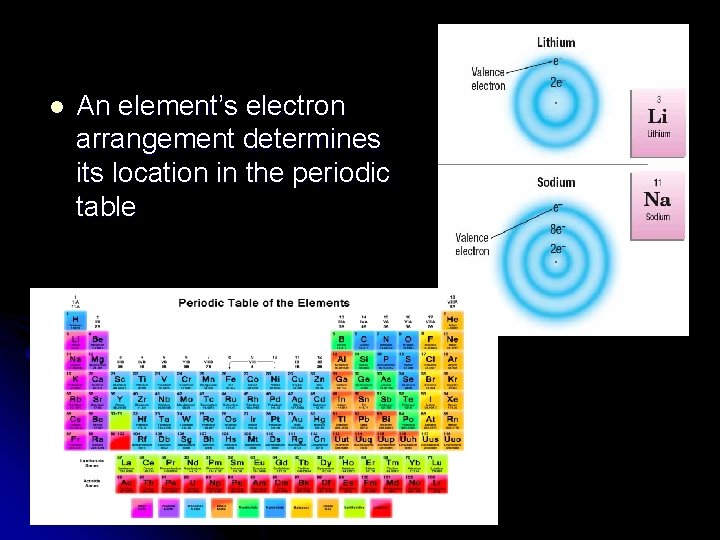
l An element’s electron arrangement determines its location in the periodic table

Ion Formation

Ions l l l l What is an ion? An atom with a charge (+ or -) Why do atoms form ions? To have a filled outermost energy level How does an atom become an ion? Atoms gain or lose e. What electron configuration does an ion possess? Electron configurations like noble gases full shell = stable

Octet Rule l Elements lose, gain, or share electrons to form the required octet in their highest remaining energy level.
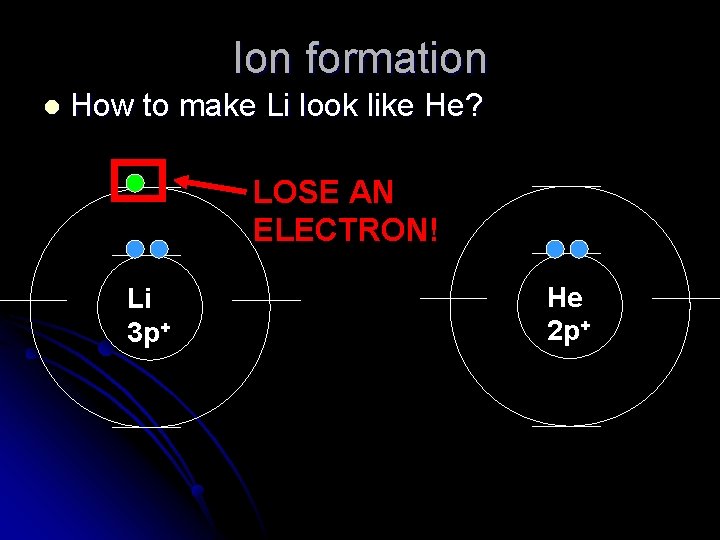
Ion formation l How to make Li look like He? LOSE AN ELECTRON! Li 3 p+ He 2 p+
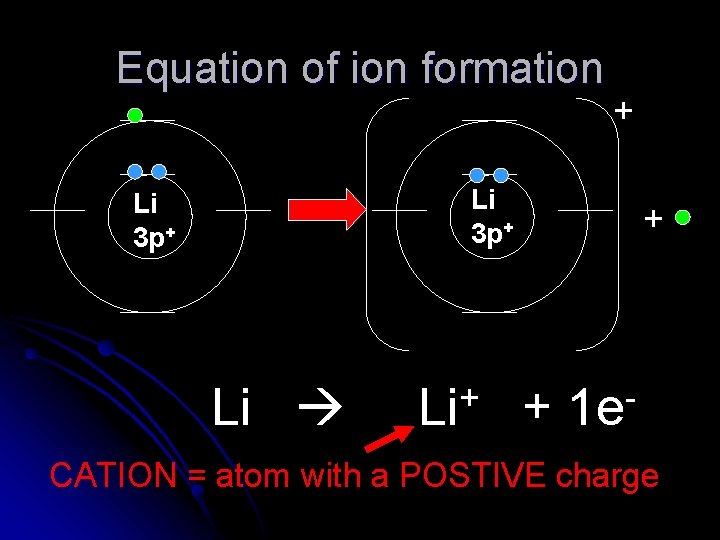
Equation of ion formation + Li 3 p+ Li + + 1 e CATION = atom with a POSTIVE charge
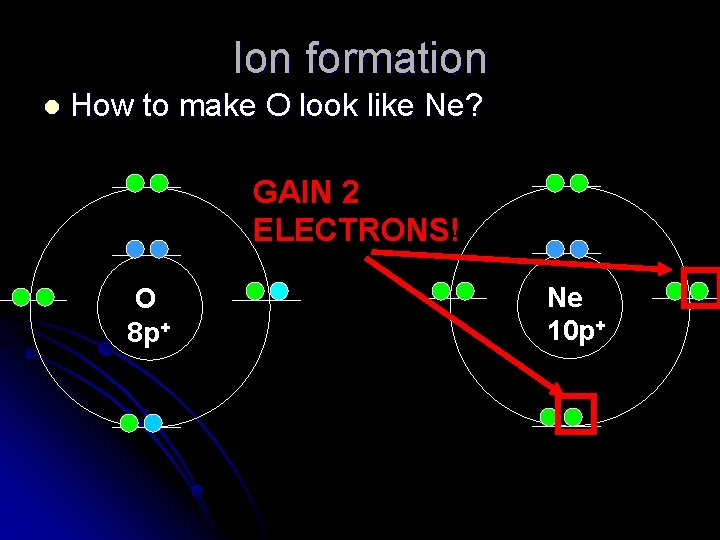
Ion formation l How to make O look like Ne? GAIN 2 ELECTRONS! O 8 p+ Ne 10 p+
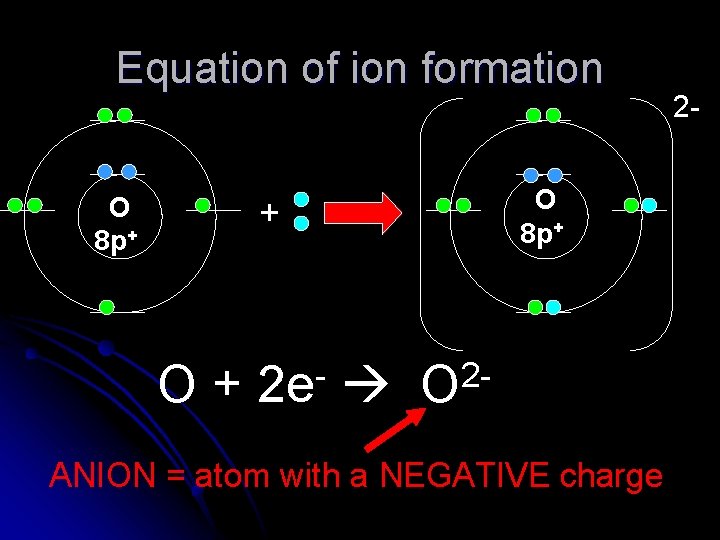
Equation of ion formation O 8 p+ + O+ 2 e 2 O ANION = atom with a NEGATIVE charge 2 -

How are Elements Classified?
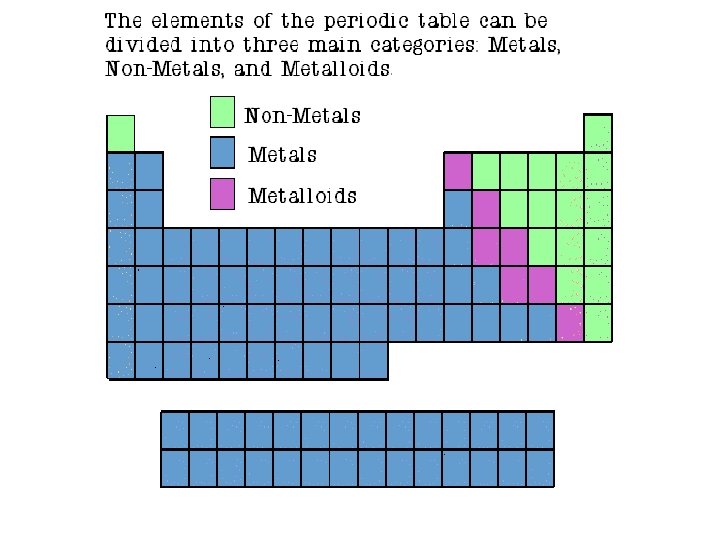

Metals l l l Most of the elements Shiny Malleable l l l can be pounded into thin sheets Conduct heat and electricity Ductile l can be stretched into thin wires

Nonmetals l l l Right side of periodic table Dull Brittle Poor conductors of heat and electricity (insulators) Not ductile or malleable Sulfur Graphite
Semiconductors l l l Metalloids Conduct electricity under certain conditions Share properties with metals and nonmetals Ductile and malleable Solids that can be shiny or dull

Learning Target Checkpoint Why do atoms form ions? l What is the difference between metals, nonmetals and metalloids? l
- Slides: 17