Section 2 e Hydrogen and water Acids And











- Slides: 16

Section 2 e Hydrogen and water

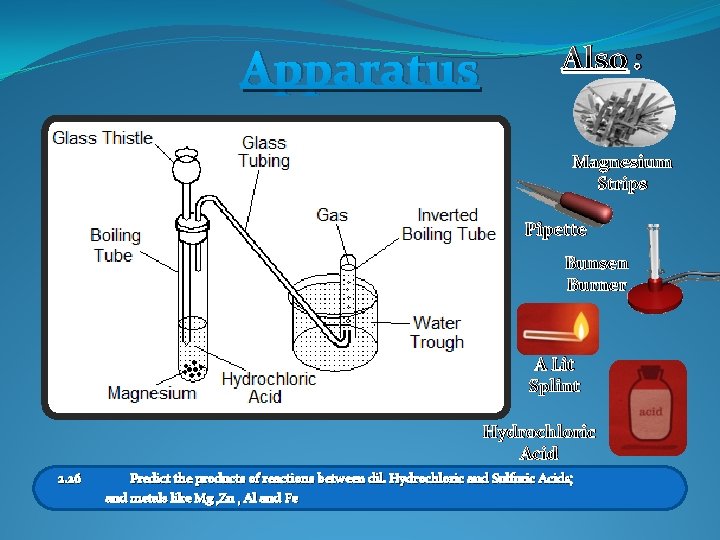
Acids And Metals Objectives 1 Describe the safety precautions necessary for the reaction of an acid with a metal 2 Know how to test for the gas released from the reaction of an acid with a metal 3 Predict the products of dil. Hydrochloric and Sulfuric Acids with various metals 2. 26 Predict the products of reactions between dil. Hydrochloric and Sulfuric Acids; and metals like Mg , Zn , Al and Fe

Acids And Metals Task 1 (Starter) Fill in the hazards and precautions you should take when carrying out the reaction between an acid and a metal SAFETY /////// 2. 26 See Worksheet For help! Predict the products of reactions between dil. Hydrochloric and Sulfuric Acids; and metals like Mg , Zn , Al and Fe

Apparatus Also : Magnesium Strips Pipette Bunsen Burner A Lit Splint Hydrochloric Acid 2. 26 Predict the products of reactions between dil. Hydrochloric and Sulfuric Acids; and metals like Mg , Zn , Al and Fe

Observations 2. 26 Predict the products of reactions between dil. Hydrochloric, Nitric and Sulfuric Acids; and metals, metal oxides and metal carbonates. (Except Nitric Acid and Metals)

Observations Hydrogen 2. 26 Predict the products of reactions between dil. Hydrochloric, Nitric and Sulfuric Acids; and metals, metal oxides and metal carbonates. (Except Nitric Acid and Metals)

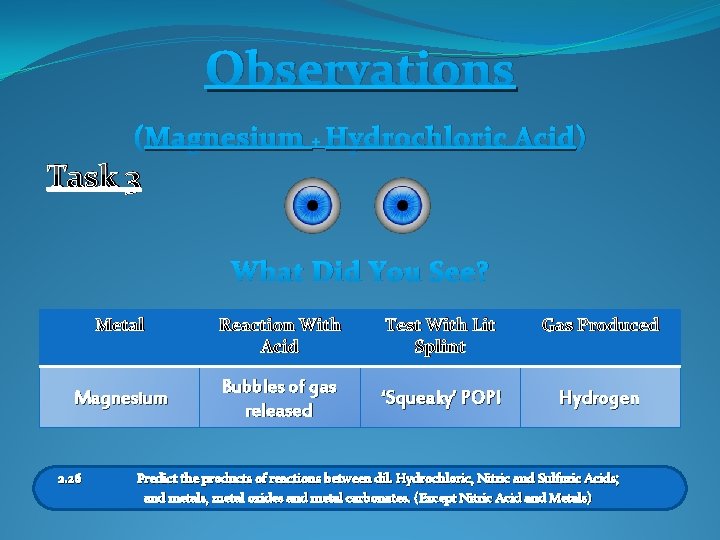
Observations (Magnesium + Hydrochloric Acid) Task 3 What Did You See? Metal Magnesium 2. 26 Reaction With Acid Test With Lit Splint Gas Produced Bubbles of gas released ‘Squeaky’ POP! Hydrogen Predict the products of reactions between dil. Hydrochloric, Nitric and Sulfuric Acids; and metals, metal oxides and metal carbonates. (Except Nitric Acid and Metals)

Objective 2. 26 �Describe the reaction of dilute HCl and H 2 SO 4 with �Mg �Al �Zn �Fe

Experiment �Take a few pieces of the metal in the test tube �Add 5 ml of HCl to it �Cover the test tube with a rubber bung �Test the gas with a lighted splint �Write down the observations in your copy book.

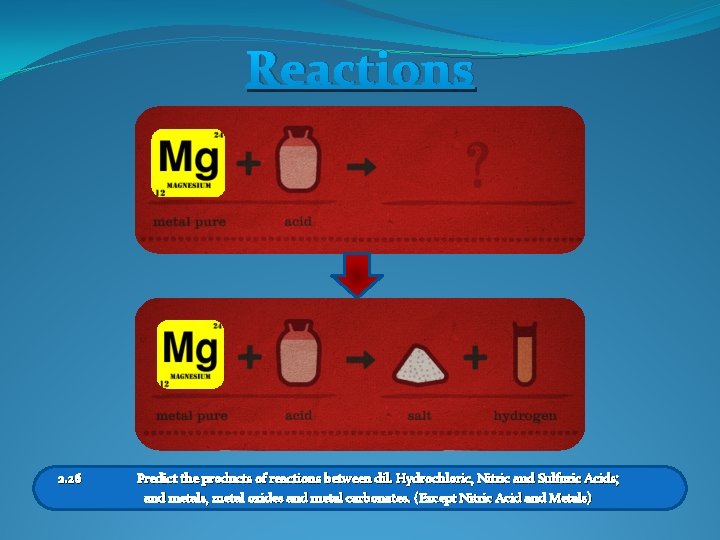
Reactions 2. 26 Predict the products of reactions between dil. Hydrochloric, Nitric and Sulfuric Acids; and metals, metal oxides and metal carbonates. (Except Nitric Acid and Metals)

Naming Products Metal + Acid M+A Salt + Hydrogen S+H Magnesium + Hydrochloric Acid Magnesium Chloride + Hydrogen 2. 26 Predict the products of reactions between dil. Hydrochloric, Nitric and Sulfuric Acids; and metals, metal oxides and metal carbonates. (Except Nitric Acid and Metals)

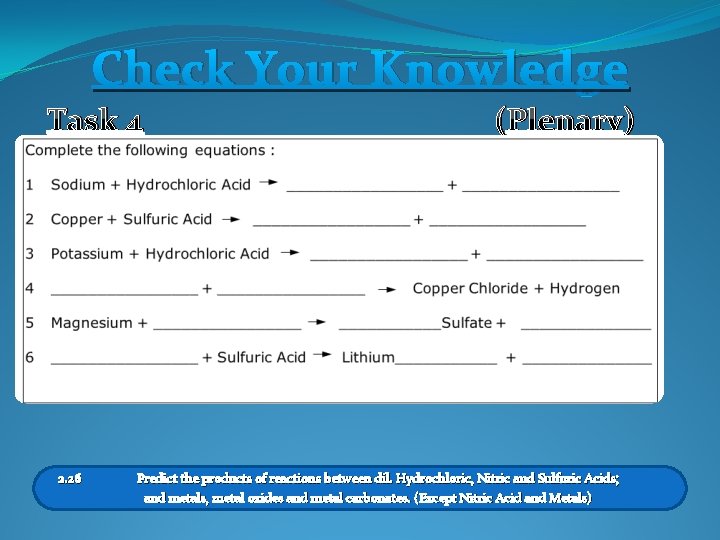
Naming Other Salts The salts formed are : Metal Salts The 1 st Part Of The Name Is From The Metal The 2 nd Part Of The Name Is From The Acid Lithium chlo ric Acid = Chloride Salts Hydrochlo Sodium Sulf uric Acid = Sulfate Salts Magnesium + Hydrochlo ric Acid Magnesium Chloride Task 4 Now try the examples on your worksheet 2. 26 Predict the products of reactions between dil. Hydrochloric, Nitric and Sulfuric Acids; and metals, metal oxides and metal carbonates. (Except Nitric Acid and Metals)

Check Your Knowledge Task 4 2. 26 (Plenary) Predict the products of reactions between dil. Hydrochloric, Nitric and Sulfuric Acids; and metals, metal oxides and metal carbonates. (Except Nitric Acid and Metals)

Combustion of hydrogen �Word equation �Hydrogen + oxygen water �Chemical equation �H 2 + O 2 H 2 O �Where do you think this reaction is used?

Chemical test for water �Anhydrous Cu. SO 4 +H 2 O Cu. SO 4. 5 H 2 O � colourless blue

Physical test for water �Melting point �Boling point �Freezing point �density