Section 2 8 Naming Simple Compounds Vocabulary Review

Section 2. 8 Naming Simple Compounds Vocabulary - Review Q: What is a cation? A: A positive ion formed by a metal Examples: Na+ Ca 2+ Al 3+ Q: What is an anion? A: A negative ion formed by a nonmetal. Examples: H- F- O 2 - P 3 Q: What is a polyatomic ion? A: A group of charged atoms NH 4+ SO 42 - Chemistry 1 2/5/2022 Return to TOC 1
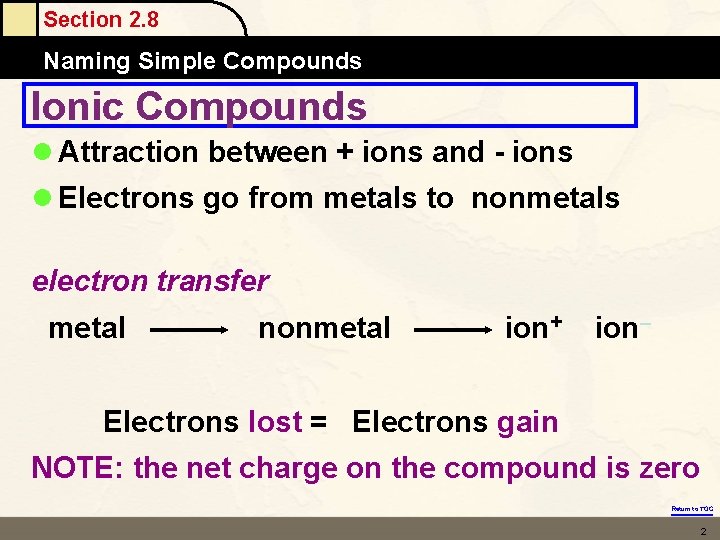
Section 2. 8 Naming Simple Compounds Ionic Compounds l Attraction between + ions and - ions l Electrons go from metals to nonmetals electron transfer metal nonmetal ion+ ion– Electrons lost = Electrons gain NOTE: the net charge on the compound is zero Return to TOC 2

Section 2. 8 Naming Simple Compounds Formation of Ionic Compounds Return to TOC 3
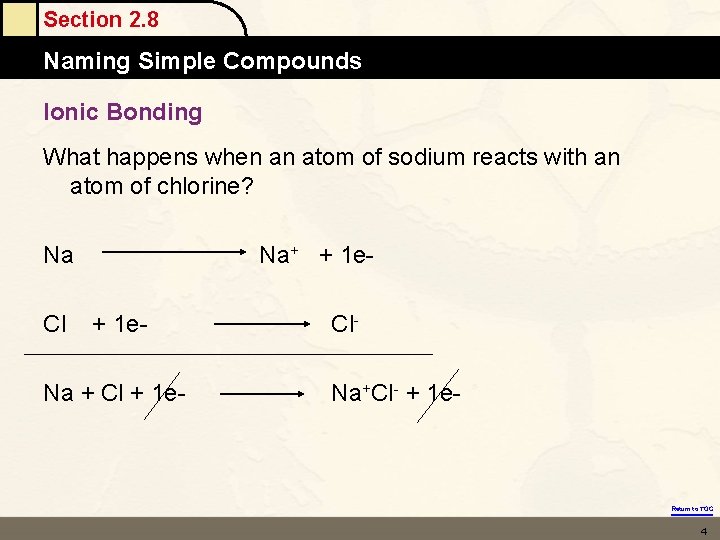
Section 2. 8 Naming Simple Compounds Ionic Bonding What happens when an atom of sodium reacts with an atom of chlorine? Na Cl Na+ + 1 e- Na + Cl + 1 e- Cl. Na+Cl- + 1 e- Return to TOC 4
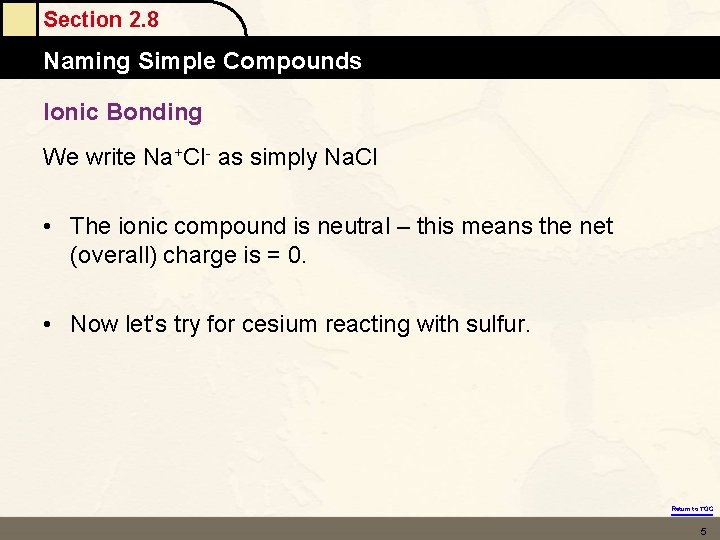
Section 2. 8 Naming Simple Compounds Ionic Bonding We write Na+Cl- as simply Na. Cl • The ionic compound is neutral – this means the net (overall) charge is = 0. • Now let’s try for cesium reacting with sulfur. Return to TOC 5
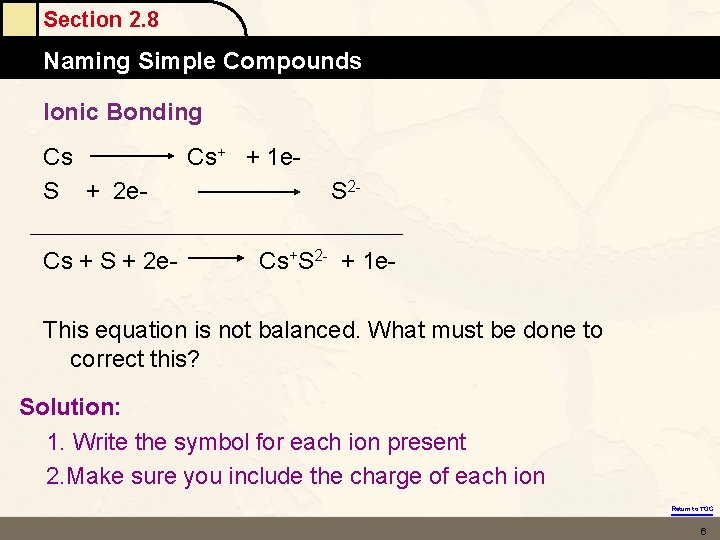
Section 2. 8 Naming Simple Compounds Ionic Bonding Cs S + 2 e. Cs + S + 2 e- Cs+ + 1 e. S 2 Cs+S 2 - + 1 e- This equation is not balanced. What must be done to correct this? Solution: 1. Write the symbol for each ion present 2. Make sure you include the charge of each ion Return to TOC 6
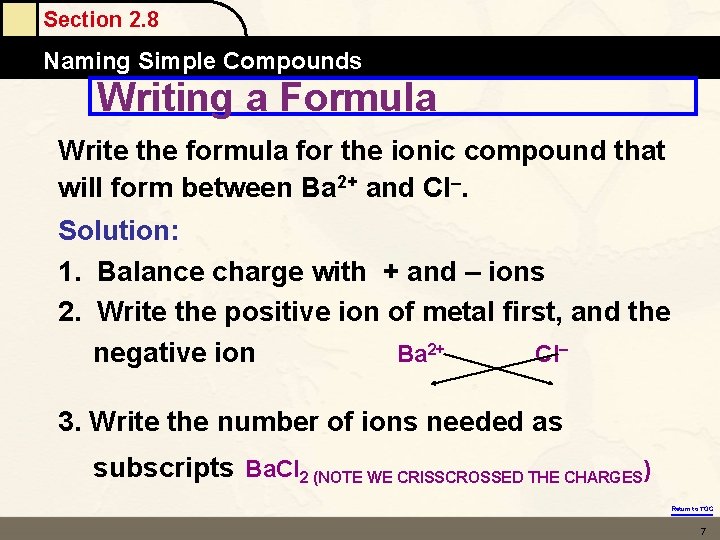
Section 2. 8 Naming Simple Compounds Writing a Formula Write the formula for the ionic compound that will form between Ba 2+ and Cl. Solution: 1. Balance charge with + and – ions 2. Write the positive ion of metal first, and the negative ion Ba 2+ Cl 3. Write the number of ions needed as subscripts Ba. Cl 2 (NOTE WE CRISSCROSSED THE CHARGES) Return to TOC 7
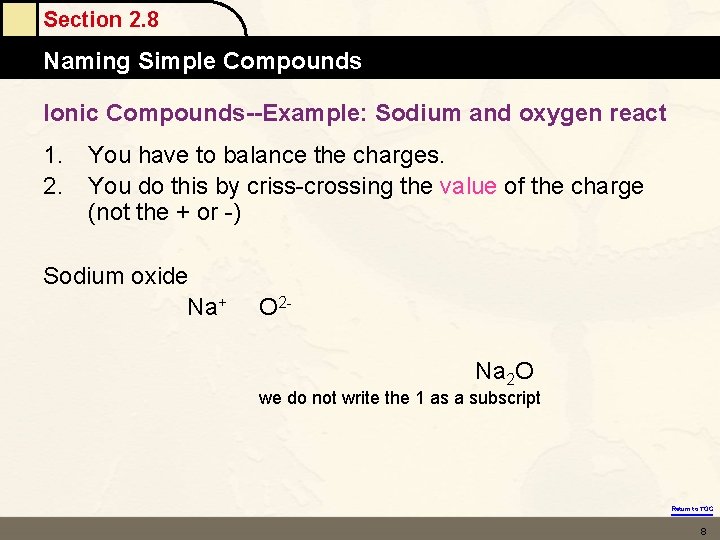
Section 2. 8 Naming Simple Compounds Ionic Compounds--Example: Sodium and oxygen react 1. 2. You have to balance the charges. You do this by criss-crossing the value of the charge (not the + or -) Sodium oxide Na+ O 2 Na 2 O we do not write the 1 as a subscript Return to TOC 8

Section 2. 8 Naming Simple Compounds Ionic Compounds Lithium iodide Li+ I – Li. I Return to TOC 9

Section 2. 8 Naming Simple Compounds Ionic Compounds Magnesium oxide Mg 2+ O 2 Mg 2 O 2 MUST be reduced to Mg. O Return to TOC 10

Section 2. 8 Naming Simple Compounds Ionic Compounds Let’s try a few: Barium nitride Ba 3 N 2 Aluminum oxide Al 2 O 3 Cesium iodide Cs. I Sodium fluoride Na. F Strontium bromide Sr. Br 2 Return to TOC 11
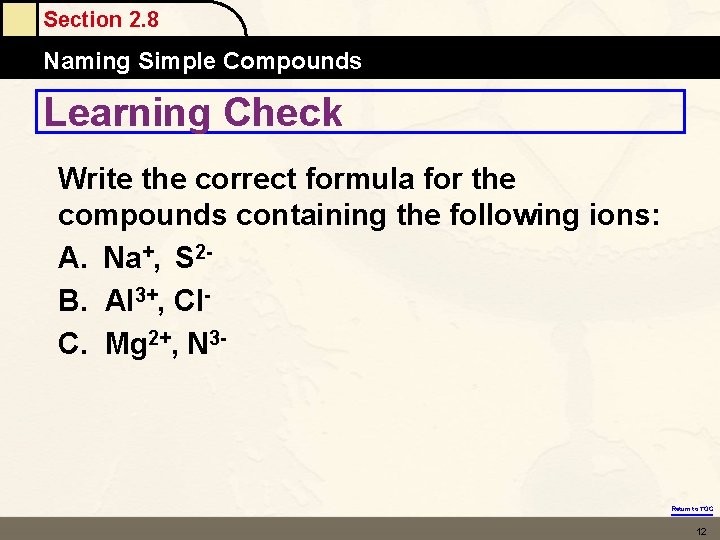
Section 2. 8 Naming Simple Compounds Learning Check Write the correct formula for the compounds containing the following ions: A. Na+, S 2 B. Al 3+, Cl. C. Mg 2+, N 3 - Return to TOC 12
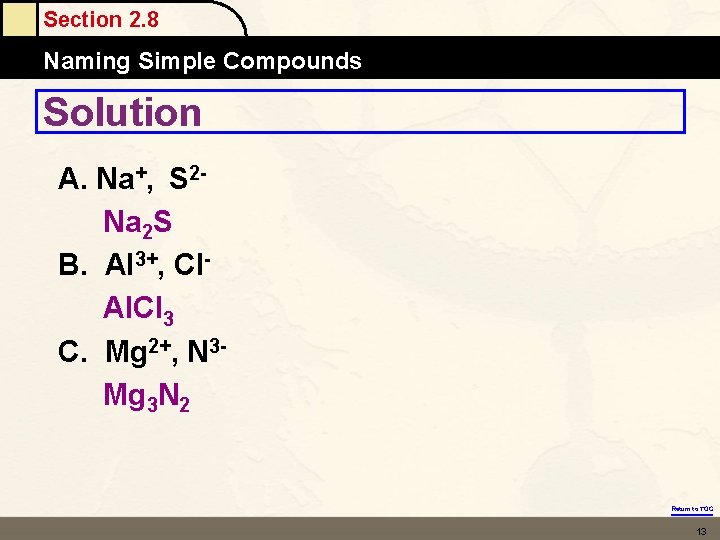
Section 2. 8 Naming Simple Compounds Solution A. Na+, S 2 Na 2 S B. Al 3+, Cl. Al. Cl 3 C. Mg 2+, N 3 Mg 3 N 2 Return to TOC 13

Section 2. 8 Naming Simple Compounds Naming Compounds • Binary Compounds § § • Binary Ionic Compounds § • Composed of two elements Ionic and covalent compounds included Metal—nonmetal Binary Covalent Compounds § Nonmetal—nonmetal Return to TOC 14

Section 2. 8 Naming Simple Compounds • Binary Compounds § • Binary Ionic Compounds § • Composed of two elements Metal—nonmetal Binary Covalent Compounds § Nonmetal—nonmetal Return to TOC 15
Section 2. 8 Naming Simple Compounds • Binary ionic compounds contain positive cations and negative anions. § Type I compounds • § Metal present forms only one cation. Type II compounds • Metal present can form 2 or more cations with different charges. Return to TOC 16

Section 2. 8 Naming Simple Compounds Binary Ionic Compounds (Type I monatomic cations & anions) 1. Contain 2 different elements 2. The cation is always named first and the anion second. 2. A cation has same name as element. Examples: Ca 2+ calcium Al 3+ aluminum Na + sodium 3. A anion has the same name as the element name with adding –ide to the end. Examples: Cl - chloride S 2 - sulfide P 3 - phosphide Return to TOC 17

Section 2. 8 Naming Simple Compounds Common Simple Cations and Anions Return to TOC 18
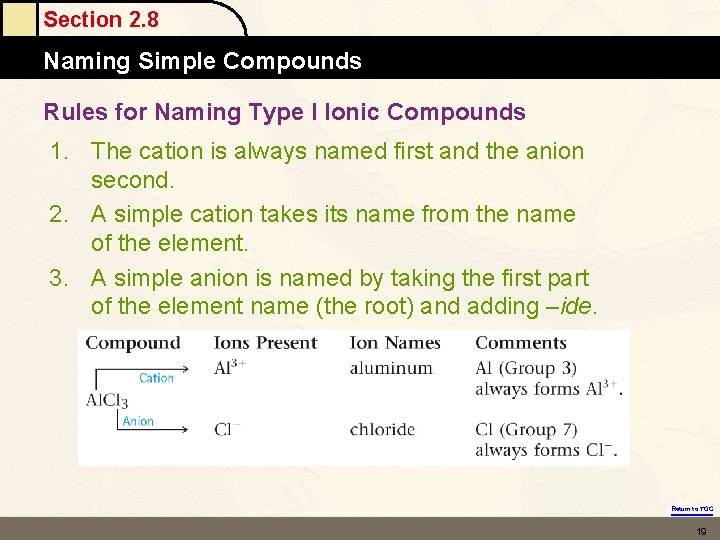
Section 2. 8 Naming Simple Compounds Rules for Naming Type I Ionic Compounds 1. The cation is always named first and the anion second. 2. A simple cation takes its name from the name of the element. 3. A simple anion is named by taking the first part of the element name (the root) and adding –ide. Return to TOC 19
Section 2. 8 Naming Simple Compounds Binary Ionic Compounds (Type I) • Examples: KCl Potassium chloride Mg. Br 2 Magnesium bromide Ca. O Calcium oxide Return to TOC 20
Section 2. 8 Naming Simple Compounds Ionic Compounds Now let us put it all together: Na 3 N Cation: Anion: sodium nitride Compound: Sodium nitride Return to TOC 21

Section 2. 8 Naming Simple Compounds Ionic Compounds Ba. O Cation: barium Anion: oxide Compound: barium oxide Return to TOC 22

Section 2. 8 Naming Simple Compounds Naming Binary Ionic Compounds (Type I) SUMMARY - Name the metal first, then the nonmetal with ending changed to -ide. Examples: Na. Cl sodium chloride Zn. I 2 zinc iodide Al 2 O 3 aluminum oxide KCl Potassium chloride Mg. Br 2 Magnesium bromide Ca. O Calcium oxide Return to TOC 23

Section 2. 8 Naming Simple Compounds Exercise What is the name of the compound Sr. Br 2? a) b) c) d) strontium bromine sulfur bromide strontium dibromide strontium bromide Return to TOC 24
Section 2. 8 Naming Simple Compounds Learning Check Complete the names of the following type I binary compounds: Na 3 N sodium ________ KBr potassium ________ Al 2 O 3 aluminum ________ Mg. S _____________ Return to TOC 25

Section 2. 8 Naming Simple Compounds Solution Complete the names of the following binary compounds: Na 3 N sodium nitride KBr potassium bromide Al 2 O 3 aluminum oxide Mg. S magnesium sulfide Return to TOC 26
Section 2. 8 Naming Simple Compounds Binary Ionic Compounds (Type II) • • Metals in these compounds can form more than one type of positive charge. Charge on the metal ion must be specified. Roman numeral indicates the charge of the metal cation. Transition metal cations usually require a Roman numeral. Return to TOC 27
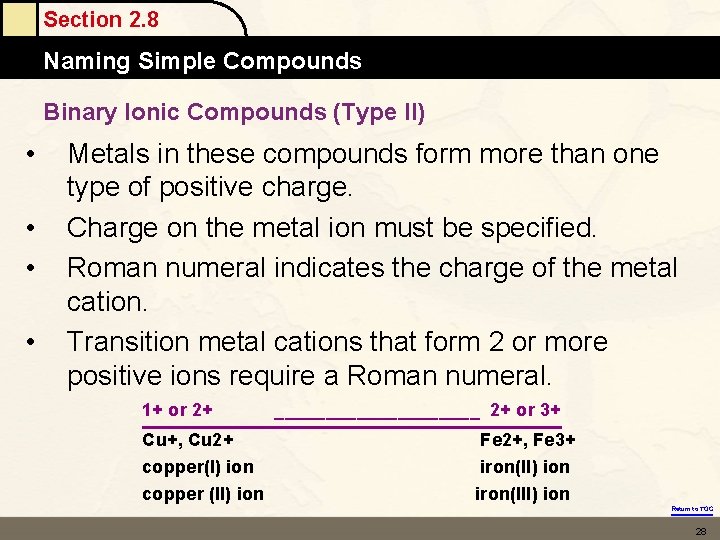
Section 2. 8 Naming Simple Compounds Binary Ionic Compounds (Type II) • • Metals in these compounds form more than one type of positive charge. Charge on the metal ion must be specified. Roman numeral indicates the charge of the metal cation. Transition metal cations that form 2 or more positive ions require a Roman numeral. 1+ or 2+ Cu+, Cu 2+ copper(I) ion copper (II) ion __________ 2+ or 3+ Fe 2+, Fe 3+ iron(II) ion iron(III) ion Return to TOC 28

Section 2. 8 Naming Simple Compounds Common Type II Cations Return to TOC 29
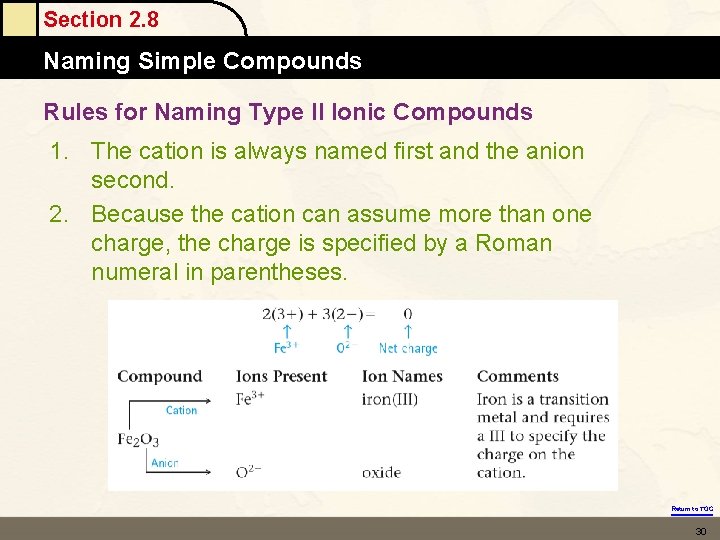
Section 2. 8 Naming Simple Compounds Rules for Naming Type II Ionic Compounds 1. The cation is always named first and the anion second. 2. Because the cation can assume more than one charge, the charge is specified by a Roman numeral in parentheses. Return to TOC 30

Section 2. 8 Naming Simple Compounds Binary Ionic Compounds (Type II) • Examples: Cu. Br Copper(I) bromide Fe. S Iron(II) sulfide Pb. O 2 Lead(IV) oxide Return to TOC 31

Section 2. 8 Naming Simple Compounds Binary Ionic Compounds (Type II) Use a roman number after the name of a metal that forms two or more ions (note compound is neutral) Example: Fe. Cl 3 Cu. Cl Sn. F 4 Pb. Cl 2 Fe 2 S 3 (Fe 3+) (Cu+ ) (Sn 4+) (Pb 2+) (Fe 3+) iron (III) chloride copper (I) chloride tin (IV) fluoride lead (II) chloride iron (III) sulfide Return to TOC 32

Section 2. 8 Naming Simple Compounds Exercise What is the name of the compound Cr. O 2? a) b) c) d) chromium oxide chromium(II) oxide chromium(IV) oxide chromium dioxide Return to TOC 33
Section 2. 8 Naming Simple Compounds Exercise What is the correct name of the compound that results from the most stable ion for sulfur and the metal ion that contains 24 electrons? a) b) c) d) iron(III) sulfide chromium(II) sulfide nickel(III) sulfate iron(II) sulfide Return to TOC 34
Section 2. 8 Naming Simple Compounds Learning Check Complete the names of the following binary compounds with variable metal ions: Fe. Br 2 iron (_____) bromide Cu 2 O copper (_____) oxide Sn. Cl 4 ___(_____ ) _______ Fe 2 O 3 ____________ Cu. S ____________ Return to TOC 35

Section 2. 8 Naming Simple Compounds Solution Complete the names of the following binary compounds with variable metal ions: Fe. Br 2 iron ( II ) bromide Cu 2 O copper ( I ) oxide Sn. Cl 4 tin (IV) chloride Fe 2 O 3 iron (III) oxide Cu. S copper (II) sulfide Return to TOC 36
Section 2. 8 Naming Simple Compounds Learning Check Name the following compounds: A. Ca. O B. Sn. Cl 4 C. Co 2 O 3 Return to TOC 37

Section 2. 8 Naming Simple Compounds Solution Name the following compounds: A. Ca. O calcium oxide B. Sn. Cl 4 tin(IV) chloride C. Co 2 O 3 cobalt (III) oxide Return to TOC 38
Section 2. 8 Naming Simple Compounds • • Polyatomic ions are charged entities composed of several atoms bound together. They have special names and must be memorized. (see Table 2. 5 on pg. 62 in text). • Examples of compounds containing polyatomic ions: Na. OH Sodium hydroxide Mg(NO ) Magnesium nitrate 32 (NH ) SO Ammonium sulfate 42 4 Return to TOC 39
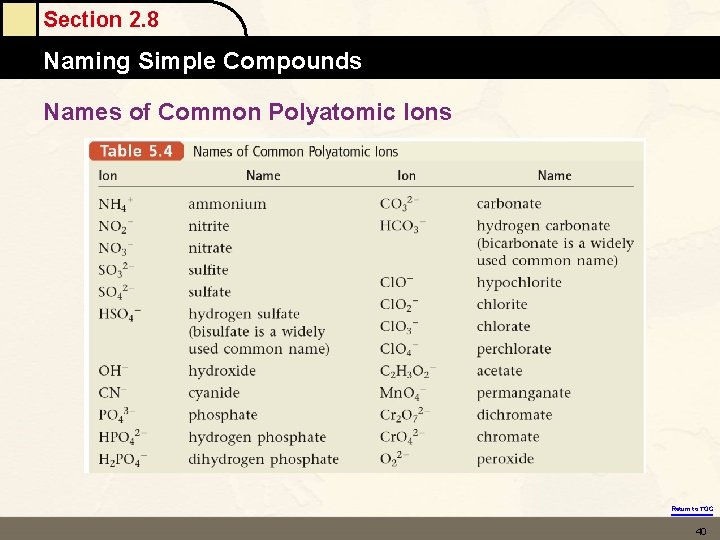
Section 2. 8 Naming Simple Compounds Names of Common Polyatomic Ions Return to TOC 40
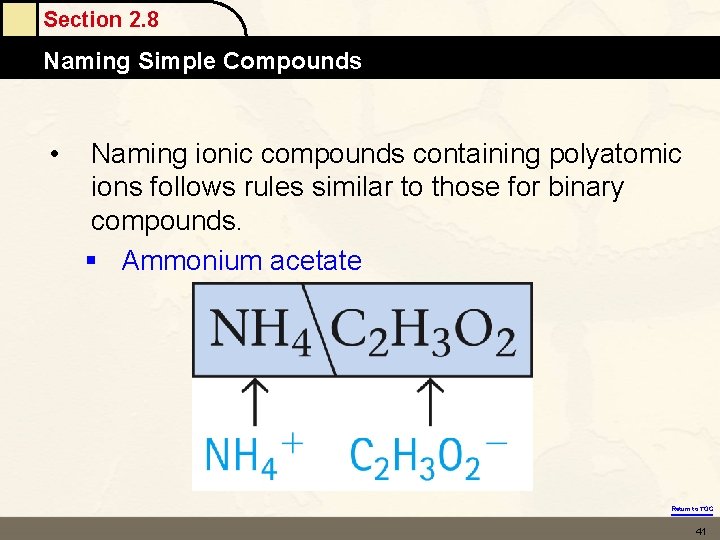
Section 2. 8 Naming Simple Compounds • Naming ionic compounds containing polyatomic ions follows rules similar to those for binary compounds. § Ammonium acetate Return to TOC 41

Section 2. 8 Naming Simple Compounds Examples Na. OH Sodium hydroxide Mg(NO 3)2 Magnesium nitrate (NH 4)2 SO 4 Ammonium sulfate Fe. PO 4 Iron(III) phosphate Return to TOC 42
Section 2. 8 Naming Simple Compounds Exercise What is the name of the compound KCl. O 3? a) b) c) d) potassium chlorite potassium chlorate potassium perchlorate potassium carbonate Return to TOC 43
- Slides: 43