Section 2 8 Naming Inorganic Compounds Chemical Nomenclature





- Slides: 15

Section 2. 8 Naming Inorganic Compounds

Chemical Nomenclature • Naming system of substances • Latin: – Nomen~ name • Two main categories: – Organic and Inorganic Calare~ to call

Inorganic Compound Rules Ionic Acids Molecular

Ionic Compounds Anions Cations

Ionic Compounds • 1. Cations – a) Same name as the metal they formed from: – Na+ sodium ion – b) Metals forming different cations: use Roman numerals in parentheses: – Fe 2+ iron (II) ion – Fe 3+ iron (III) ion ~ Older method: -ous or –ic ending Fe 2+ ferrous ion Fe 3+ ferric ion

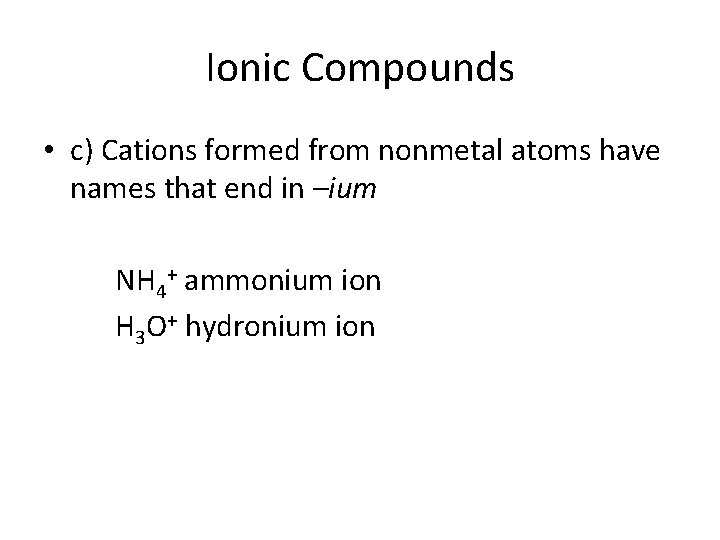
Ionic Compounds • c) Cations formed from nonmetal atoms have names that end in –ium NH 4+ ammonium ion H 3 O+ hydronium ion

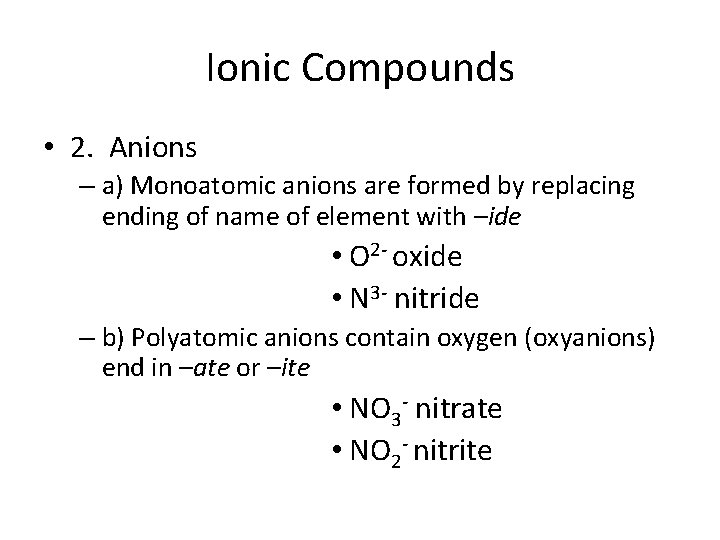
Ionic Compounds • 2. Anions – a) Monoatomic anions are formed by replacing ending of name of element with –ide • O 2 - oxide • N 3 - nitride – b) Polyatomic anions contain oxygen (oxyanions) end in –ate or –ite • NO 3 - nitrate • NO 2 - nitrite

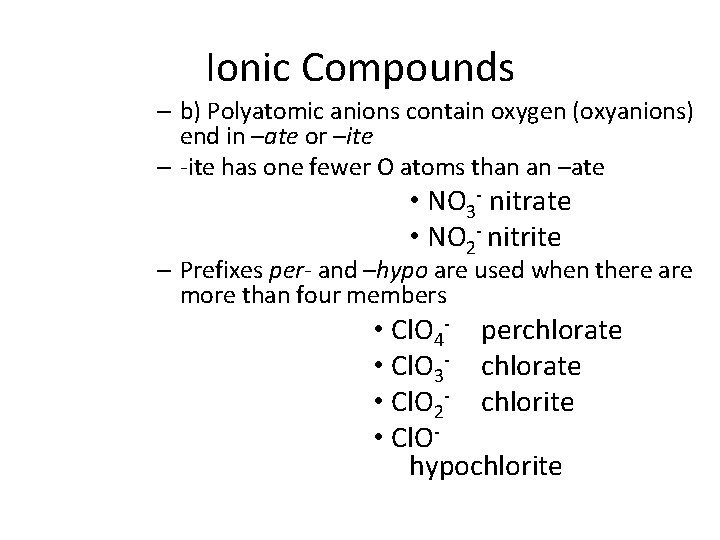
Ionic Compounds – b) Polyatomic anions contain oxygen (oxyanions) end in –ate or –ite – -ite has one fewer O atoms than an –ate • NO 3 - nitrate • NO 2 - nitrite – Prefixes per- and –hypo are used when there are more than four members • Cl. O 4 - perchlorate • Cl. O 3 - chlorate • Cl. O 2 - chlorite • Cl. Ohypochlorite

Ionic Compounds • c) Anions derived from adding H+ to an oxyanion: begin with either hydrogen or dihydrogen » CO 32» HCO 3 - carbonate hydrogen carbonate » PO 43 - phosphate » H 2 PO 43 dihydrogen phosphate

Ionic Compounds • 3. Compounds – Names consist of the cation name followed by the anion name • Ca. Cl 2 • Al. PO 3 • Cu. SO 4 calcium chloride aluminum phosphite copper (II) sulfate

Acids -ides -ates /-ites

Acids • 1. With anions ending in –ide: change –ide to – ic, add the prefix hydro-, and follow with the word acid Anion Corresponding Acid Cl- (chloride) HCl (hydrochloric acid) S 2 - (sulfide) H 2 S (hydrosulfuric acid

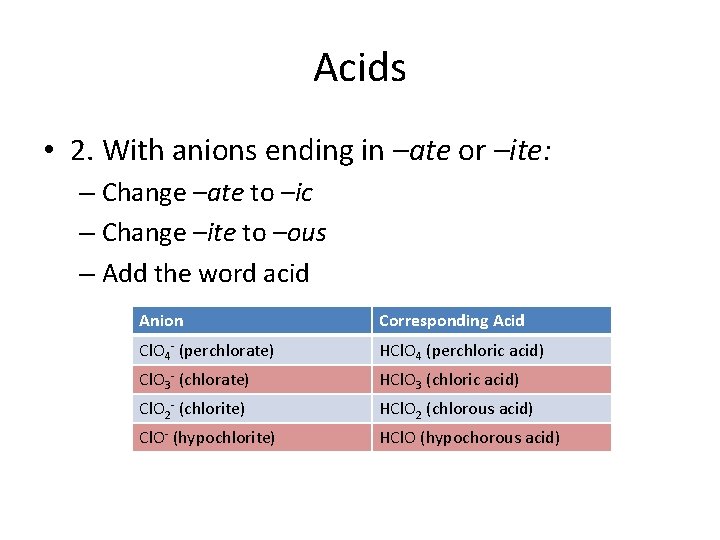
Acids • 2. With anions ending in –ate or –ite: – Change –ate to –ic – Change –ite to –ous – Add the word acid Anion Corresponding Acid Cl. O 4 - (perchlorate) HCl. O 4 (perchloric acid) Cl. O 3 - (chlorate) HCl. O 3 (chloric acid) Cl. O 2 - (chlorite) HCl. O 2 (chlorous acid) Cl. O- (hypochlorite) HCl. O (hypochorous acid)

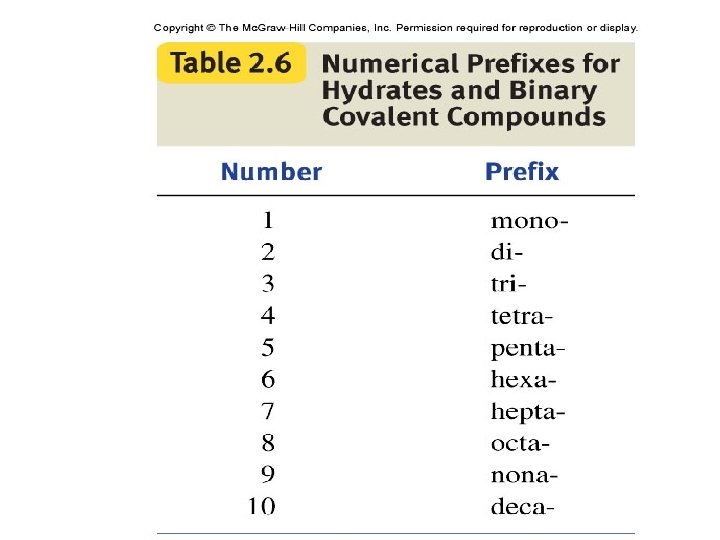
Molecular Compounds (Binary) • 1. Name the element farther to the left in the periodic table first. • 2. If both in same group, the one with the higher atomic number is named first. • 3. Name the second with an –ide ending • 4. Greek prefixes are used to indicate number of atoms of each element
