Section 2 4 2 5 Enzymes Chemical Reactions





- Slides: 16

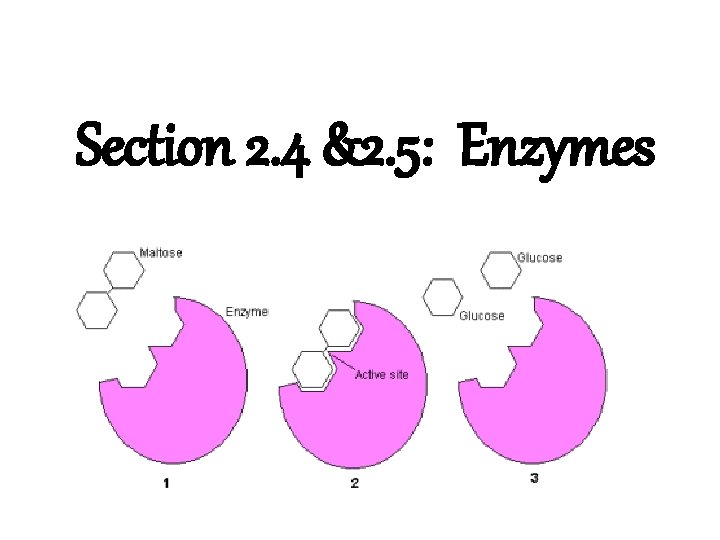
Section 2. 4 &2. 5: Enzymes

Chemical Reactions Release and Absorb Energy • Energy is neither created or destroyed, it continues to be transferred. Potential Energy Potential to Kinetic Energy Potential Energy • All Chemical reactions involve a change/transfer in energy.

Observing a Chemical Reaction 1. Put 50 m. L of water into a flask 2. Add 10 drops of Bromothymol Blue (an acid indicator which will turn the solution yellow if an acid is present) 3. Get the temperature in `C. 4. Add one Alka Seltzer tablet (split into two) 5. Observe reaction and write down observations 6. Get the temperature after the reaction occurs. What you observed was: CO 2 + H 2 O H 2 CO 3 (Carbonic Acid) Reactants Product

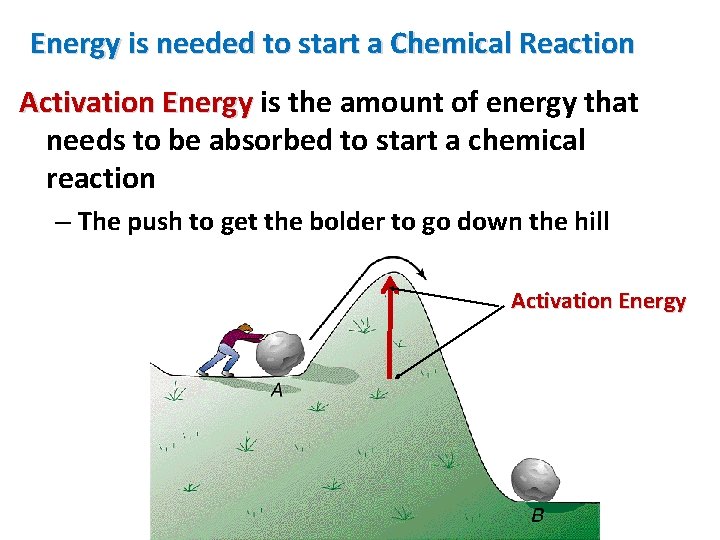
Energy is needed to start a Chemical Reaction Activation Energy is the amount of energy that needs to be absorbed to start a chemical reaction – The push to get the bolder to go down the hill Activation Energy

How can we reduce the amount of activation energy needed to get a reaction started? 2 H 2 O 2 2 H 2 O + O 2 – This reaction will occur on its own but will take a long time and a lot of activation energy – Catalysts reduce the amount of activation energy necessary to get a reaction started – This is a method of cleaning contact lenses using a Manganese dioxide disk which will quickly break hydrogen peroxide into water in oxygen and get your contacts totally disinfected!! • The Manganese dioxide disk can be used over and over again without a loss of functioning.

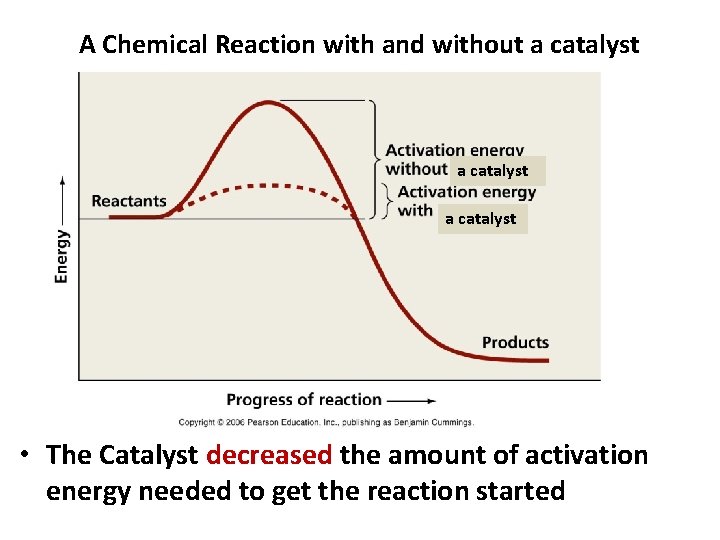
A Chemical Reaction with and without a catalyst • The Catalyst decreased the amount of activation energy needed to get the reaction started

Does anyone suffer from eating…? …Beans, peanuts, broccoli, cauliflower, brussel sprouts, cabbage, peppers, onions…? • You know what I am talking about!!! • This happens because we can’t digest alpha-galactosidose present in these foods • There is something to prevent this!! • Beano contains the enzyme alpha-galactosidase to allow us to break down alpha-galactosidose

Maybe you can’t tolerate lactose sugar and are lactose intolerant. What do people do who are lactose intolerant? They take Lactaid pills which contain the enzyme Lactase Enzymes are protein catalysts made by living organisms that reduce the amount of activation energy necessary to start and control the rate of a chemical reaction

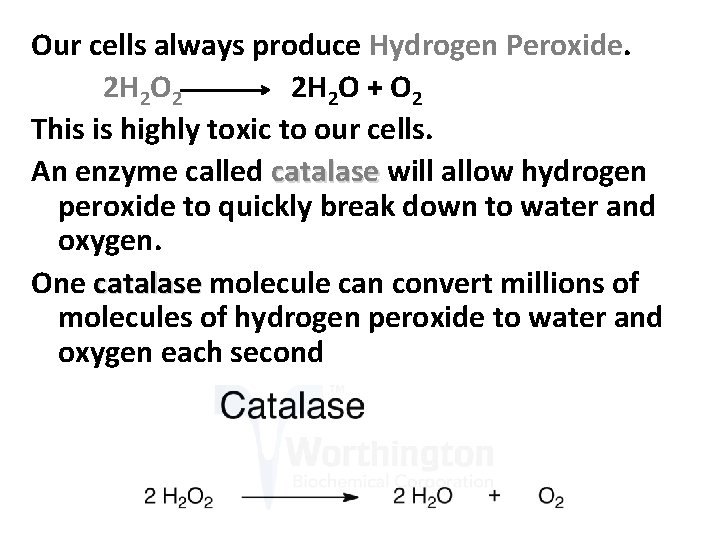
Our cells always produce Hydrogen Peroxide. 2 H 2 O 2 2 H 2 O + O 2 This is highly toxic to our cells. An enzyme called catalase will allow hydrogen peroxide to quickly break down to water and oxygen. One catalase molecule can convert millions of molecules of hydrogen peroxide to water and oxygen each second

Characteristics of Enzymes 1. Made up of Proteins 2. Are specific to whatever they act upon 3. Are reusable – don’t get used up in a chemical reaction 4. Usually have names ending in “ase” 5. Enter directly into a reaction

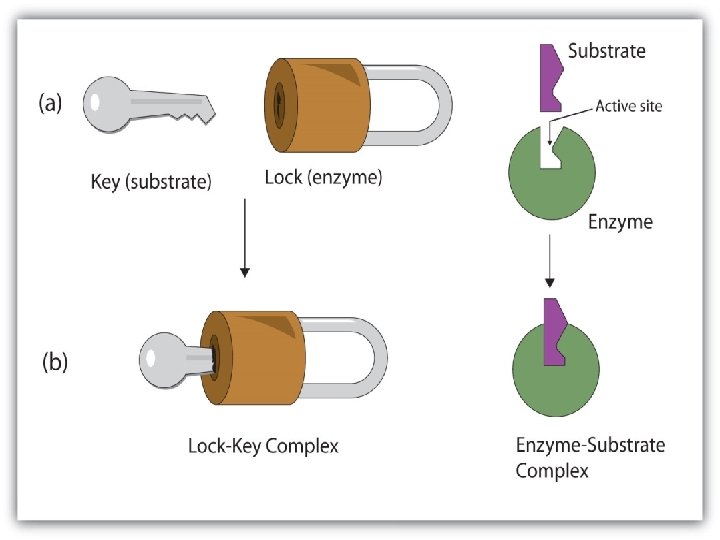
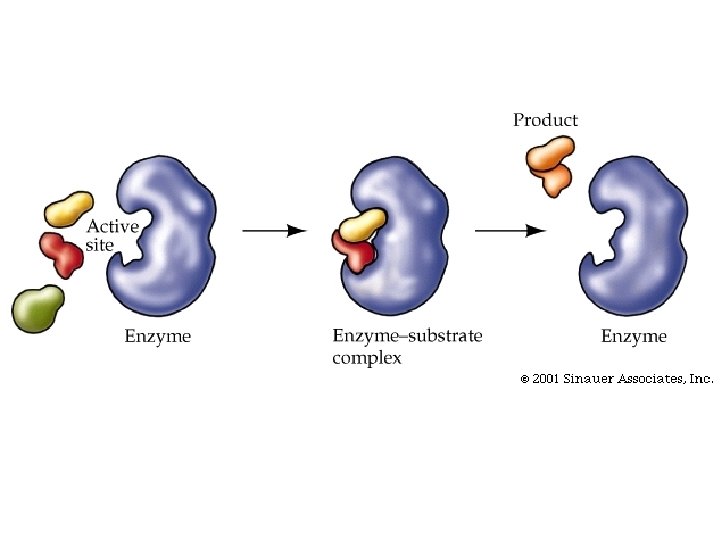
How Enzymes work • Their shape is specific to what they act upon (the substrate) substrate • They will bind directly to the substrate as an enzyme-substrate complex • The binding site is called the Active Site • A substrate and its enzyme fit together like puzzle pieces – Lock and Key Theory



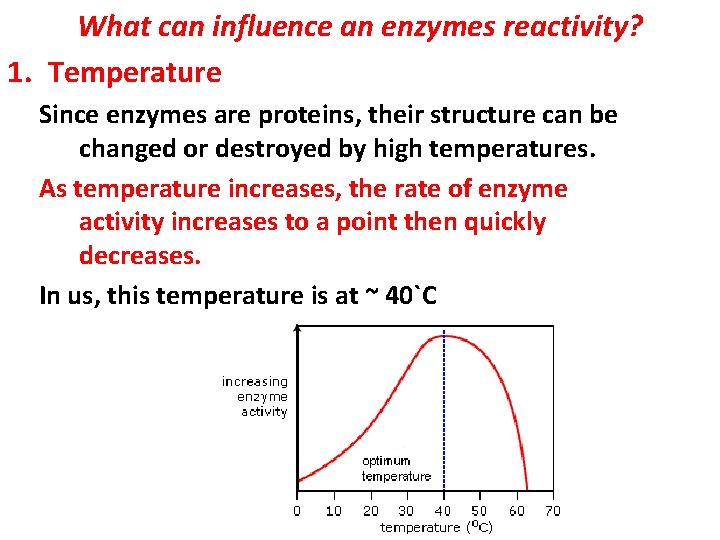
What can influence an enzymes reactivity? 1. Temperature Since enzymes are proteins, their structure can be changed or destroyed by high temperatures. As temperature increases, the rate of enzyme activity increases to a point then quickly decreases. In us, this temperature is at ~ 40`C

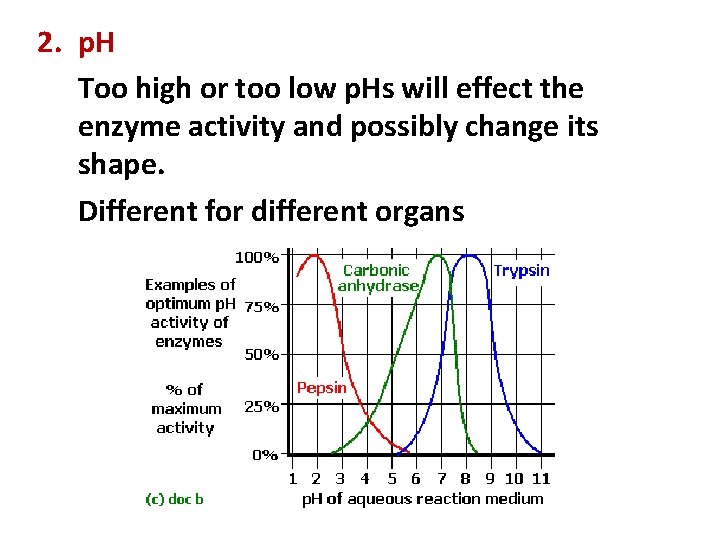
2. p. H Too high or too low p. Hs will effect the enzyme activity and possibly change its shape. Different for different organs

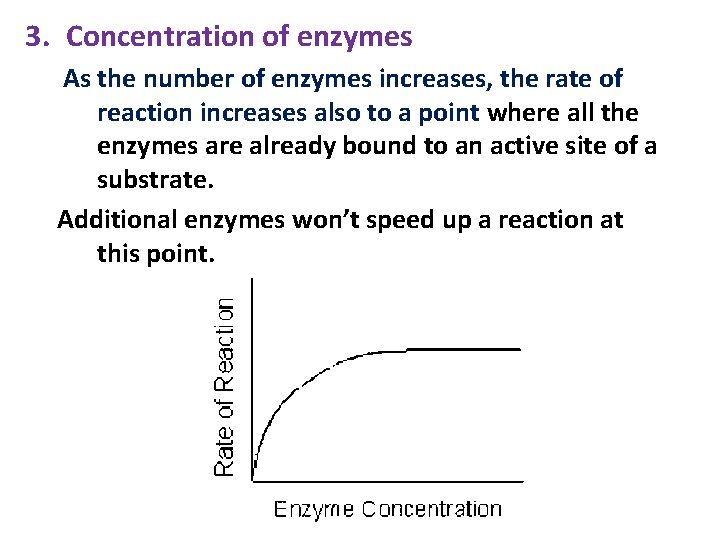
3. Concentration of enzymes As the number of enzymes increases, the rate of reaction increases also to a point where all the enzymes are already bound to an active site of a substrate. Additional enzymes won’t speed up a reaction at this point.