SECTION 2 3 CARBON COMPOUNDS Organic Chemistry Study
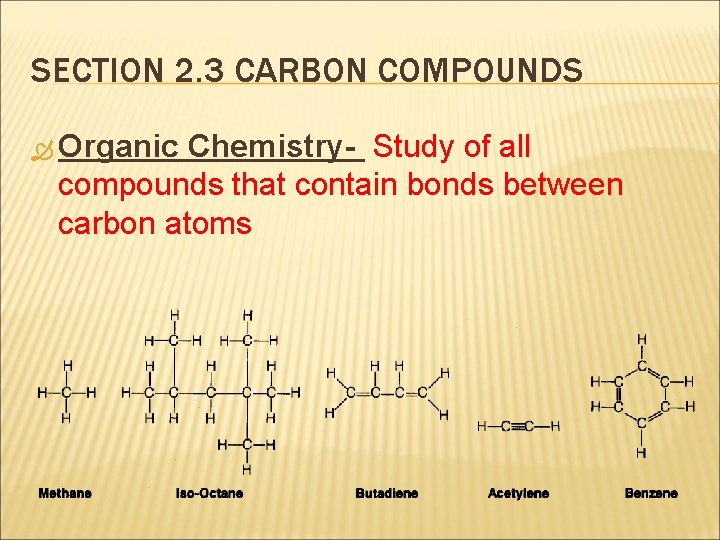
SECTION 2. 3 CARBON COMPOUNDS Organic Chemistry- Study of all compounds that contain bonds between carbon atoms
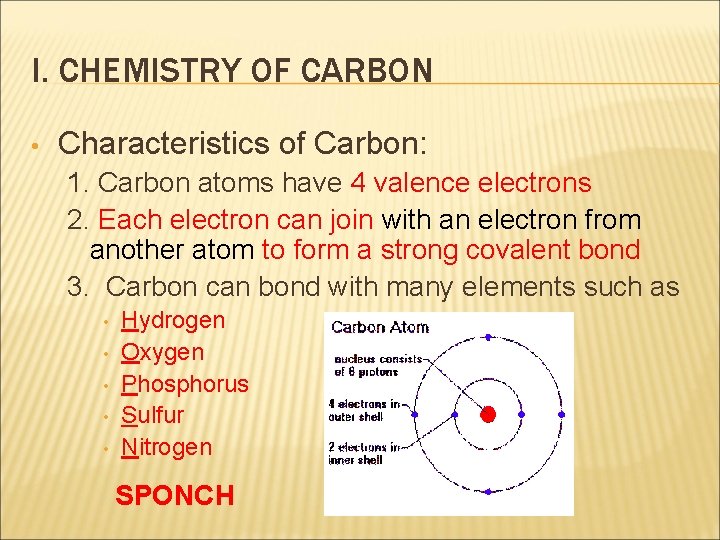
I. CHEMISTRY OF CARBON • Characteristics of Carbon: 1. Carbon atoms have 4 valence electrons 2. Each electron can join with an electron from another atom to form a strong covalent bond 3. Carbon can bond with many elements such as • • • Hydrogen Oxygen Phosphorus Sulfur Nitrogen SPONCH
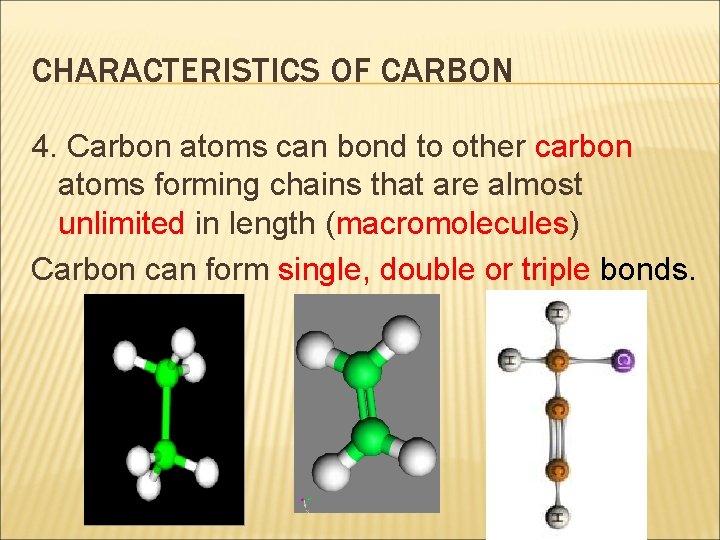
CHARACTERISTICS OF CARBON 4. Carbon atoms can bond to other carbon atoms forming chains that are almost unlimited in length (macromolecules) Carbon can form single, double or triple bonds.
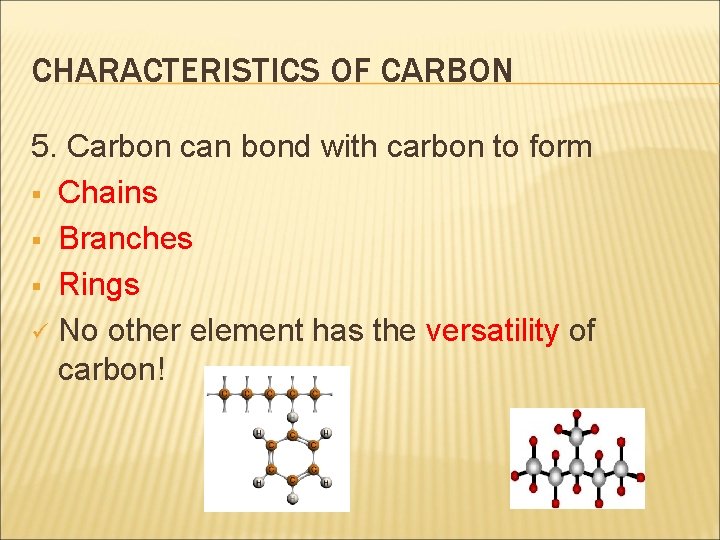
CHARACTERISTICS OF CARBON 5. Carbon can bond with carbon to form § Chains § Branches § Rings ü No other element has the versatility of carbon!
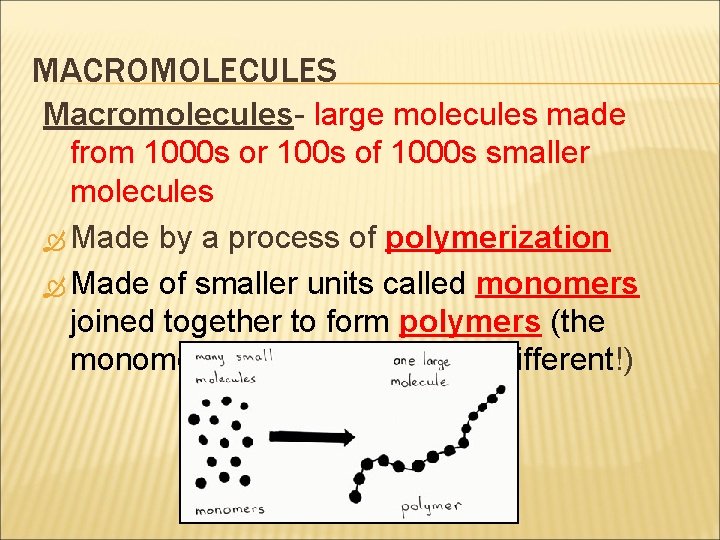
MACROMOLECULES Macromolecules- large molecules made from 1000 s or 100 s of 1000 s smaller molecules Made by a process of polymerization Made of smaller units called monomers joined together to form polymers (the monomers can be identical or different!)

ORGANIC COMPOUNDS OR BIOMOLECULES ARE CLASSIFIED INTO 4 GROUPS: 1. 2. 3. 4. Carbohydrates Lipids Nucleic Acids Proteins

1. CARBOHYDRATES Living things use carbohydrates as their main source of energy and structural purposes ü The breakdown of sugars, such as glucose (C 6 H 12 O 6) supplies immediate energy for all cell activity ü

1. CARBOHYDRATES Extra sugar is stored as complex carbohydrates known as starches ü Three types of carbohydrates: ü a) Monosaccharide- single sugar molecules • glucose (blood sugar) • galactose (milk) • fructose (fruits)
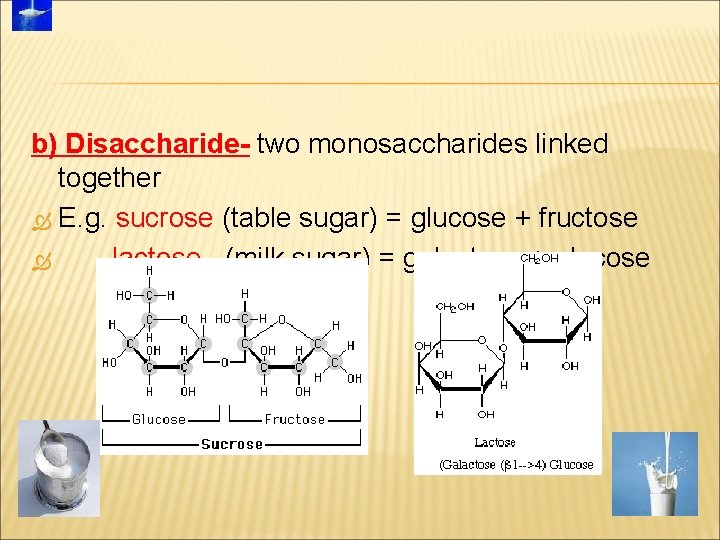
b) Disaccharide- two monosaccharides linked together E. g. sucrose (table sugar) = glucose + fructose lactose (milk sugar) = galactose + glucose
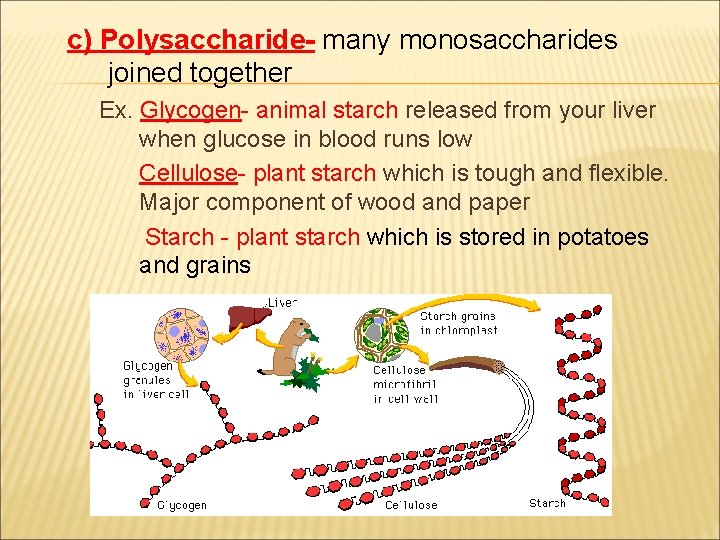
c) Polysaccharide- many monosaccharides joined together Ex. Glycogen- animal starch released from your liver when glucose in blood runs low Cellulose- plant starch which is tough and flexible. Major component of wood and paper Starch - plant starch which is stored in potatoes and grains

2. LIPIDS q The common categories of lipids are fats, oils, and waxes q Compounds made mostly from carbon and hydrogen (very few oxygen atoms) q NOT soluble in water! (hydrophobic) q Used to store energy

2. LIPIDS. Biological roles: q energy storage q insulation: especially prominent in some mammals and birds (penguins and seals). q padding of fat) (some organs have extra padding
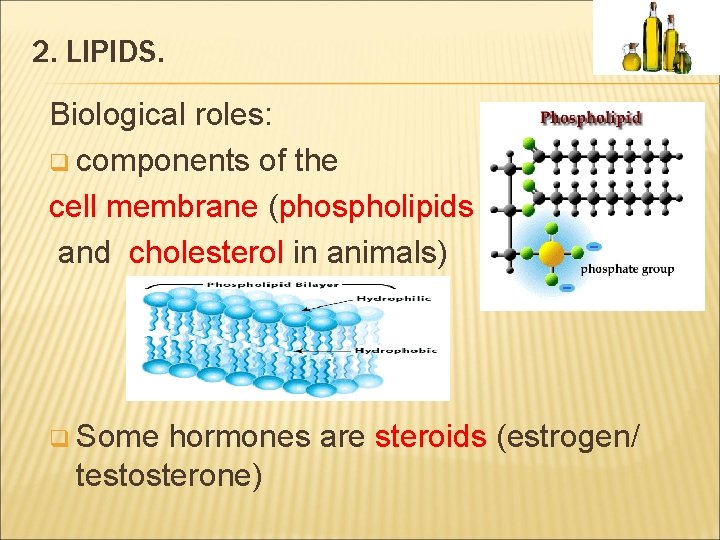
2. LIPIDS. Biological roles: q components of the cell membrane (phospholipids and cholesterol in animals) q Some hormones are steroids (estrogen/ testosterone)
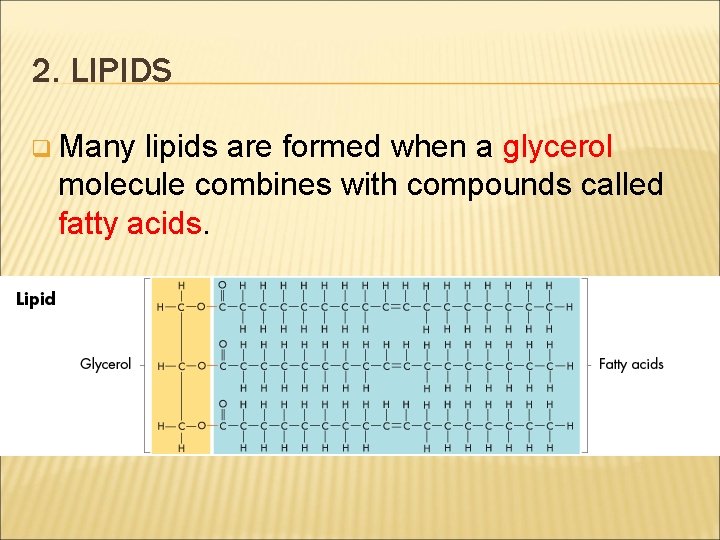
2. LIPIDS q Many lipids are formed when a glycerol molecule combines with compounds called fatty acids.
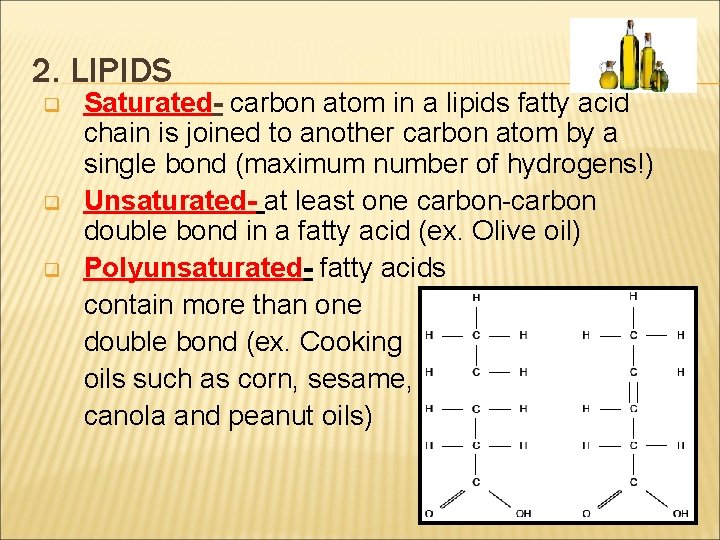
2. LIPIDS q q q Saturated- carbon atom in a lipids fatty acid chain is joined to another carbon atom by a single bond (maximum number of hydrogens!) Unsaturated- at least one carbon-carbon double bond in a fatty acid (ex. Olive oil) Polyunsaturated- fatty acids contain more than one double bond (ex. Cooking oils such as corn, sesame, canola and peanut oils)

3. NUCLEIC ACIDS q Macromolecules that contain hydrogen, oxygen, nitrogen, carbon and phosphorus q Nucleotide- individual monomer consisting of three parts: • • • a 5 -carbon sugar, a phosphate group, a nitrogenous base (A, T, C, or G)
3. NUCLEIC ACIDS q Individual nucleotides can be joined to form a nucleic acid q Nucleic acids store and transmit heredity or genetic information 1. 2. DNA- deoxyribonucleic acid, double stranded, (sugar=deoxyribose); stores genetic information RNA- ribonucleic acid, single stranded, (sugar = ribose), makes proteins
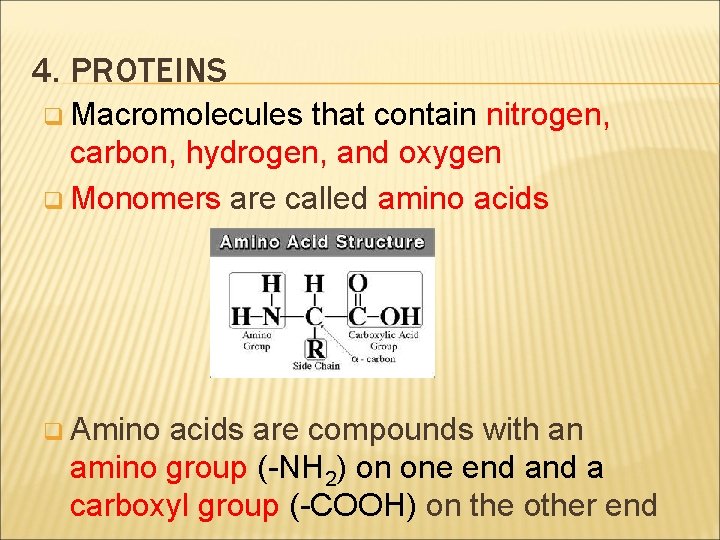
4. PROTEINS q Macromolecules that contain nitrogen, carbon, hydrogen, and oxygen q Monomers are called amino acids q Amino acids are compounds with an amino group (-NH 2) on one end a carboxyl group (-COOH) on the other end
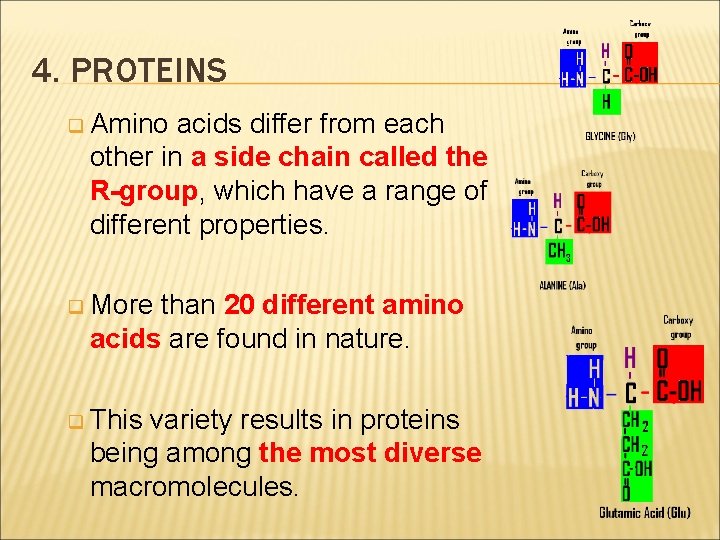
4. PROTEINS q Amino acids differ from each other in a side chain called the R-group, which have a range of different properties. q More than 20 different amino acids are found in nature. q This variety results in proteins being among the most diverse macromolecules.
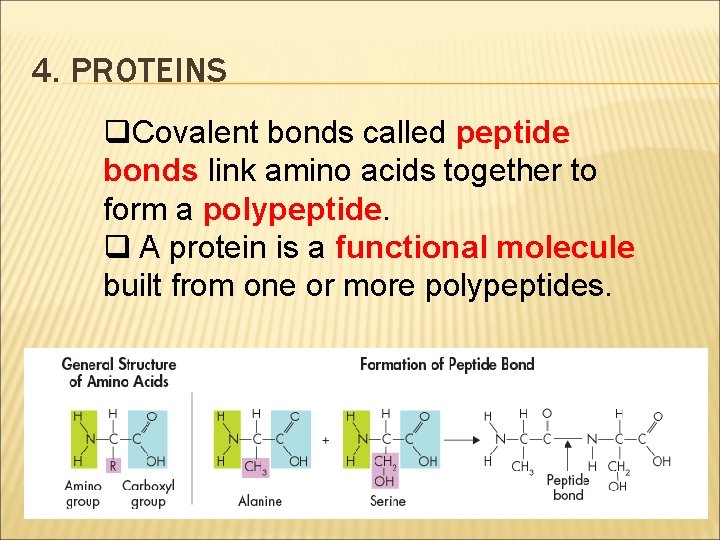
4. PROTEINS q. Covalent bonds called peptide bonds link amino acids together to form a polypeptide. q A protein is a functional molecule built from one or more polypeptides.
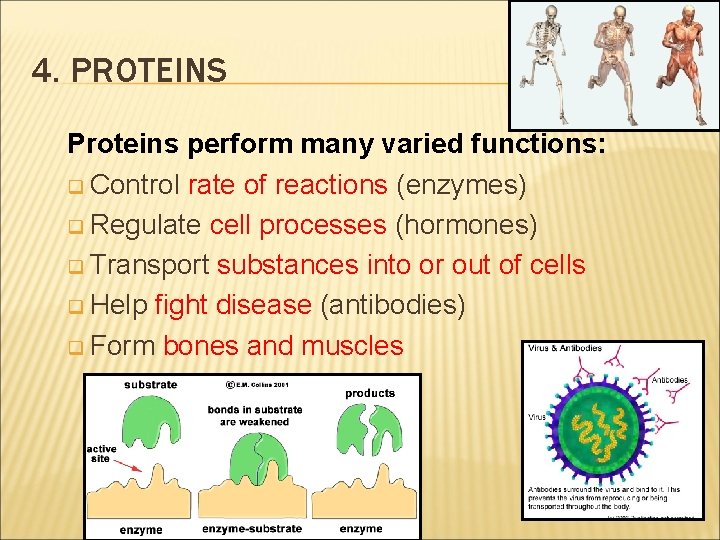
4. PROTEINS Proteins perform many varied functions: q Control rate of reactions (enzymes) q Regulate cell processes (hormones) q Transport substances into or out of cells q Help fight disease (antibodies) q Form bones and muscles
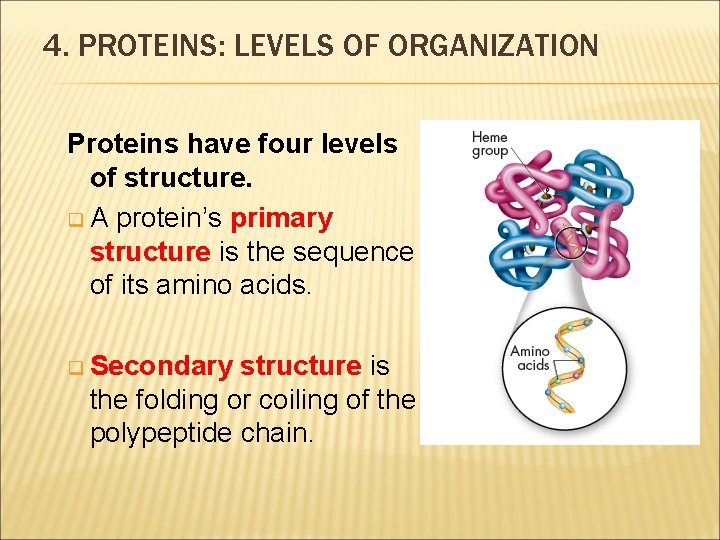
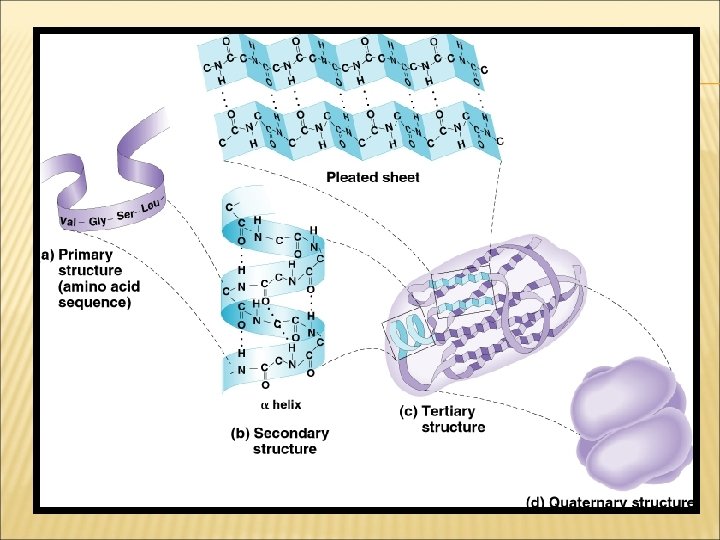
4. PROTEINS: LEVELS OF ORGANIZATION Proteins have four levels of structure. q A protein’s primary structure is the sequence of its amino acids. q Secondary structure is the folding or coiling of the polypeptide chain.
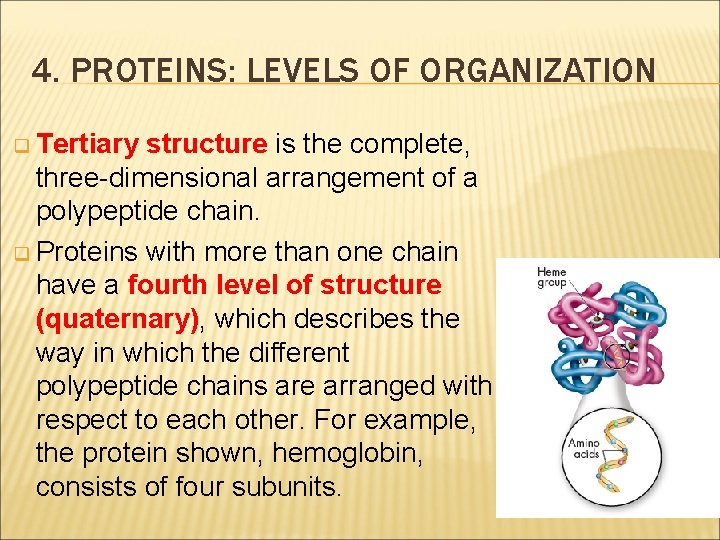
4. PROTEINS: LEVELS OF ORGANIZATION q Tertiary structure is the complete, three-dimensional arrangement of a polypeptide chain. q Proteins with more than one chain have a fourth level of structure (quaternary), which describes the way in which the different polypeptide chains are arranged with respect to each other. For example, the protein shown, hemoglobin, consists of four subunits.
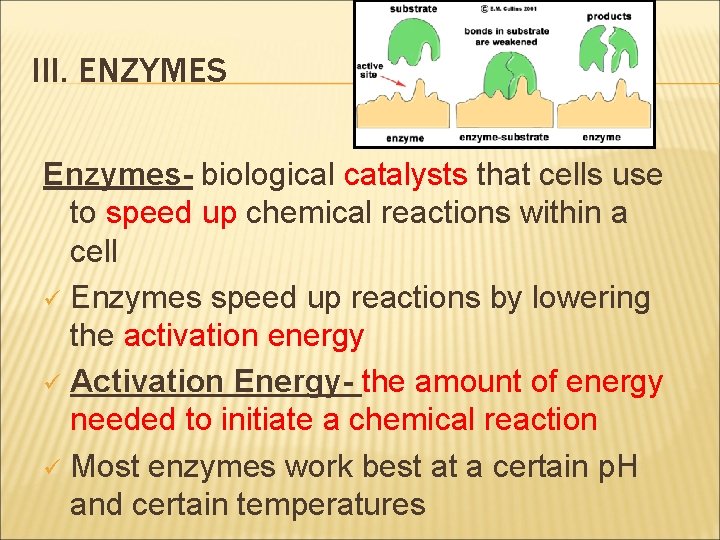
III. ENZYMES Enzymes- biological catalysts that cells use to speed up chemical reactions within a cell ü Enzymes speed up reactions by lowering the activation energy ü Activation Energy- the amount of energy needed to initiate a chemical reaction ü Most enzymes work best at a certain p. H and certain temperatures

ENZYMES ü Enzymes play essential roles in i. iii. iv. Regulating chemical pathways Making materials that cells need Releasing energy Transferring information
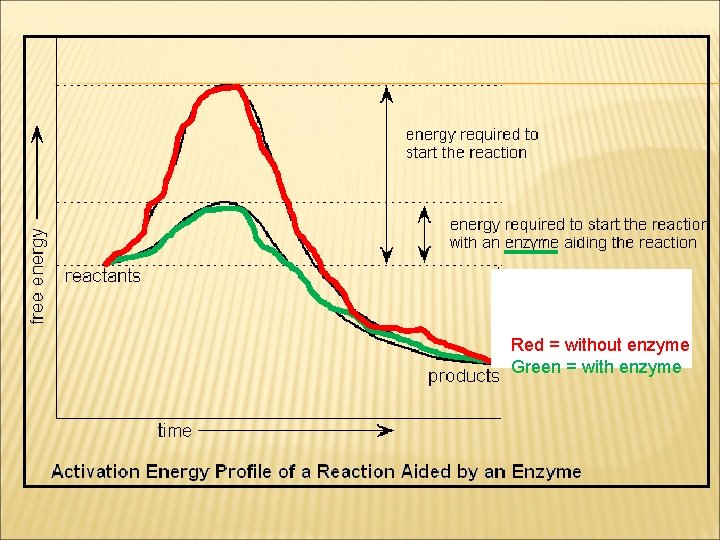
Red = without enzyme Green = with enzyme
- Slides: 27