Section 15 3 Colligative Properties Colligative Properties Physical















- Slides: 23

Section 15. 3 Colligative Properties

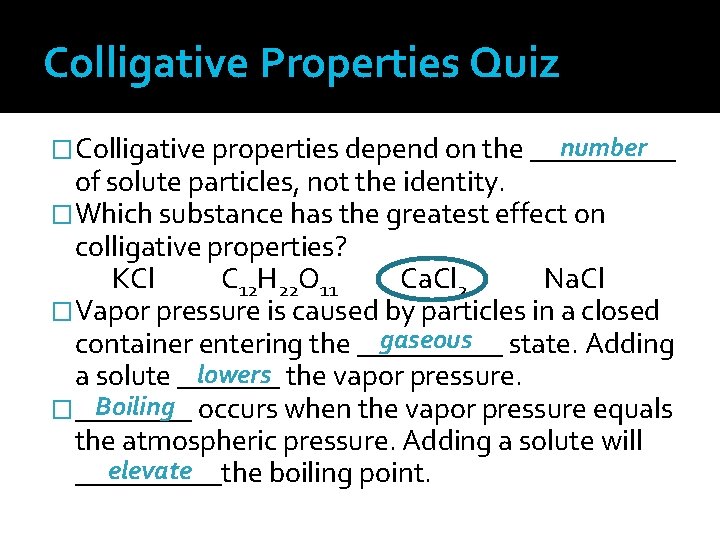
Colligative Properties Physical properties of solutions that depend on the number – not the identity – of solute particles dissolved The greater the number of particles dissolved in solution, the greater the effect on colligative properties

Electrolytes Ionic compounds dissociate in water when forming a solution The solutions formed conduct electric currents These compounds are called electrolytes Some molecular compounds also ionize in aqueous solution and are called electrolytes

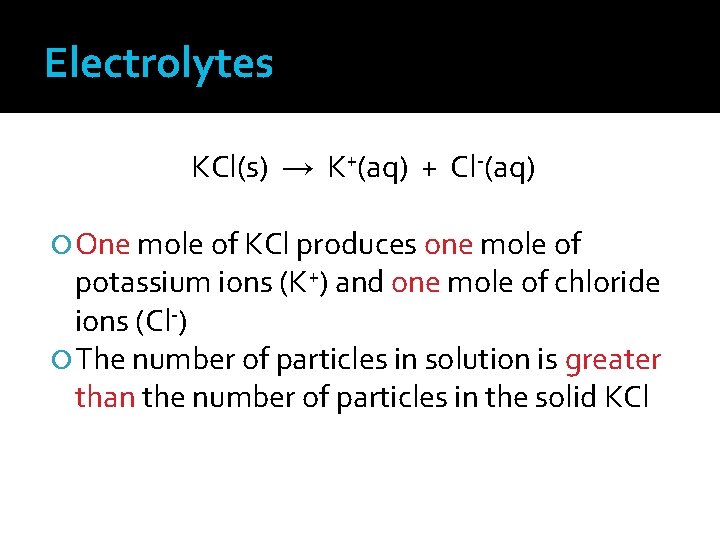
Electrolytes KCl(s) → K+(aq) + Cl-(aq) One mole of KCl produces one mole of potassium ions (K+) and one mole of chloride ions (Cl-) The number of particles in solution is greater than the number of particles in the solid KCl

Electrolytes Strong electrolytes ionize completely and produce many ions in solution Weak electrolytes only partially ionize

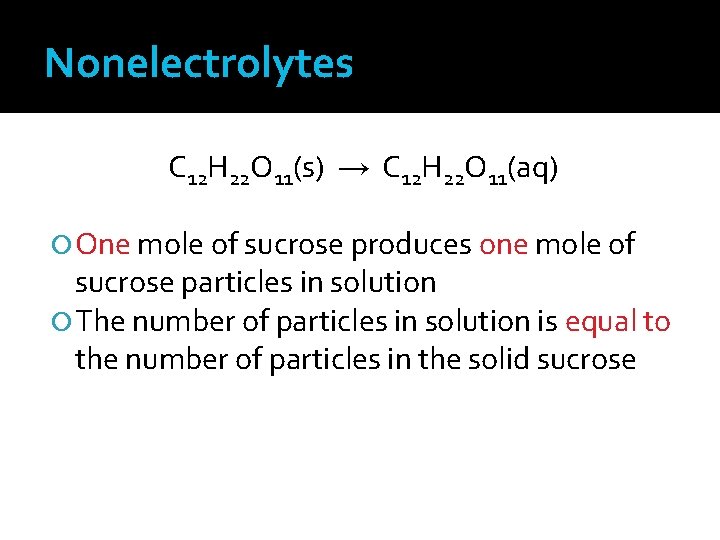
Nonelectrolytes Many molecular compounds dissolve but do not form ions in solution They form solutions that do not conduct electric currents These substances are known as nonelectrolytes One mole of a nonelectrolyte will form only one mole of particles in solution

Nonelectrolytes C 12 H 22 O 11(s) → C 12 H 22 O 11(aq) One mole of sucrose produces one mole of sucrose particles in solution The number of particles in solution is equal to the number of particles in the solid sucrose

Greatest Effect? Which would have the greatest effect on Al. Br 3 colligative properties? Mg(NO 3)2 → Mg+2(aq) + 2 NO 3 -(aq) Three moles of particles in solution C 6 H 12 O 6(s) → C 6 H 12 O 6(aq) One mole of particles in solution Al. Br 3(s) → Al+3(aq) + 3 Br-(aq) Four moles of particles in solution HCl(s) → H+(aq) + Cl-(aq) Two moles of particles in solution

Colligative Properties Vapor Pressure Lowering Vapor pressure: pressure exerted by liquid particles that have escaped the liquid’s surface and entered gaseous state When only water molecules occupy the surface, any water molecule with enough energy will evaporate

Colligative Properties Vapor Pressure Lowering In a solution, the surface area of the liquid is occupied by solute and solvent particles, decreasing area from which water can escape Fewer water molecules leave solution, resulting in lower vapor pressure

Colligative Properties Vapor Pressure Lowering Vapor pressure is a colligative property because it changes due to the addition of solute particles With more solute particles in solution, fewer water molecules at the surface escape into the gaseous state Thus, the vapor pressure decreases

Colligative Properties Boiling Point Elevation Boiling point: the temperature at which vapor pressure of substance equals atmospheric pressure; substance can move to gaseous state Boiling point of water is 100°C Adding solute lowers the vapor pressure for solution Solution particles must have more energy to vaporize or boil

Colligative Properties Boiling Point Elevation More kinetic energy is necessary Heating to a higher temperature provides the energy necessary to raise the vapor pressure to atmospheric pressure Boiling point elevation is the temperature difference between the boiling point of pure solvent and that of solution Colligative property because the greater the number of particles in solution , the greater the boiling point elevation

Colligative Properties Quiz number �Colligative properties depend on the _____ of solute particles, not the identity. �Which substance has the greatest effect on colligative properties? KCl C 12 H 22 O 11 Ca. Cl 2 Na. Cl �Vapor pressure is caused by particles in a closed gaseous state. Adding container entering the _____ lowers the vapor pressure. a solute _______ Boiling occurs when the vapor pressure equals �____ the atmospheric pressure. Adding a solute will elevate _____the boiling point.

Colligative Properties Freezing Point Depression Freezing point: the temperature at which particles do not have sufficient energy to overcome interparticle attraction and move into the organized structure of solid state Adding solute interferes with attractive forces and prevents freezing Temperature must be lowered even more to freeze

Colligative Properties Freezing Point Depression Freezing point depression: temperature difference between freezing point of pure solvent and of solution Practical Applications of Freezing Point Depression De-icing highways with calcium chloride or magnesium chloride during winter Using rock salt (mineral form of sodium chloride) with ice to make ice cream

Colligative Properties Osmotic Pressure Osmosis: diffusion of solvent particles through a semipermeable membrane from an area of high solvent concentration to lower solvent concentration A semipermeable membrane is a thin sheet of a substance with pores, which acts as a barrier allowing some particles, but not others, to cross

Colligative Properties Osmotic Pressure When a dilute solution (or pure solvent) is separated from a concentrated solution by a semipermeable membrane, water molecules (solvent) move from the dilute solution to the concentrated one but solute particles do not Dilute Solution low solute/high solvent H 2 O semipermeable membrane Concentrated Solution high solute/low solvent

Colligative Properties Osmotic Pressure Osmotic pressure is the additional pressure caused by water molecules moving across the semipermeable barrier Colligative property because it depends upon the number of solute particles in a specified volume of solution

Colligative Properties Quiz depress/lower the �Adding a solute will ________ freezing point. �Practical applications of this colligative property include: De-icing highways � ____________________ Making ice cream �Diffusion of solvent particles across a osmosis semipermeable membrane is called _____. dilute solution to a Particles move from a _______ concentrated _______solution. �Pressure caused by water moving across the semipermeable membrane is known as osmotic pressure ________

Colligative Properties Respond to three questions at bottom of page 12 in Solutions Notes. Questions should be completed for Unit 8 Notebook Check

GO-GO-MO Topic My Info About Topic A Friend’s Info (GO) A Different Friend’s Info (MO) 1. Homogeneous mixtures 2. Solvation process 3. Factors affecting solvation Your GO-GO-MO Table should have three completed entries.

GO-GO-MO Topic My Info About Topic A Friend’s Info (GO) A Different Friend’s Info (MO) 4. Like dissolves like 5. Solution concentration 6. Molarity 7. Preparing molar solutions 8. Colligative properties Add the next five topics to your table as indicated above.