Section 14 1 Intermolecular Forces and Phase Changes












- Slides: 20

Section 14. 1 Intermolecular Forces and Phase Changes What makes something a solid, liquid or a gas? Two factors determine whether a substance is a solid, liquid or a gas 1) The kinetic energies of the particles 2) The attractive inter-molecular forces between particles that tend to draw the particles together.

Section 14. 1 Intermolecular Forces and Phase Changes Kinetic Energy- Energy of motion • If the average kinetic energy is greater than the attractive forces between the particles, a substance will not condense to form a liquid or a solid. • If the kinetic energy is less than the attractive forces, a liquid or solid will form. • The Kinetic energy of different particles (solid, liquid, gas) are explained by the Kinetic Molecular Theory

Section 14. 1 Intermolecular Forces and Phase Changes Intermolecular forces in liquids and solids • The attractive forces between particles in a solid or liquid are more effective than those between particles in a gas. These forces are called intermolecular forces.

Section 14. 1 Intermolecular Forces and Phase Changes • This attraction between particles is caused by one of the following kinds of intermolecular forces: – dipole-dipole forces (Occurs in polar covalent compounds) – Hydrogen bonding (Special kind of dipole – dipole, also occurs in polar covalent compounds) – London dispersion forces (occurs in non polar covalent compounds and atoms)

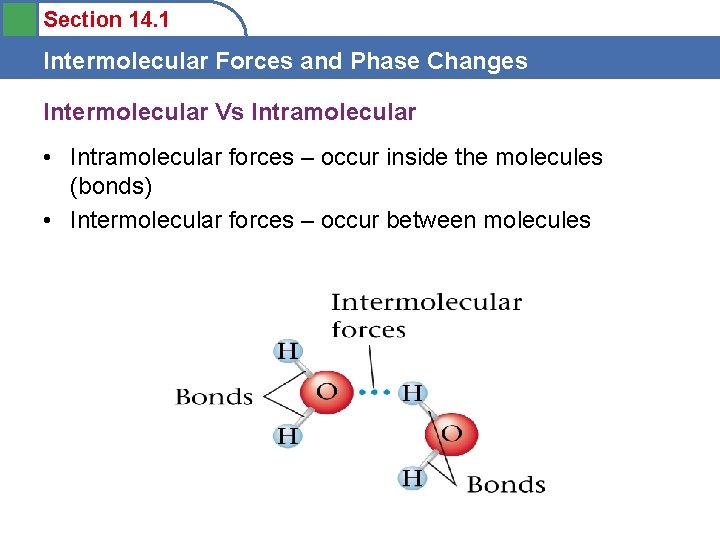
Section 14. 1 Intermolecular Forces and Phase Changes Intermolecular Vs Intramolecular • Intramolecular forces – occur inside the molecules (bonds) • Intermolecular forces – occur between molecules

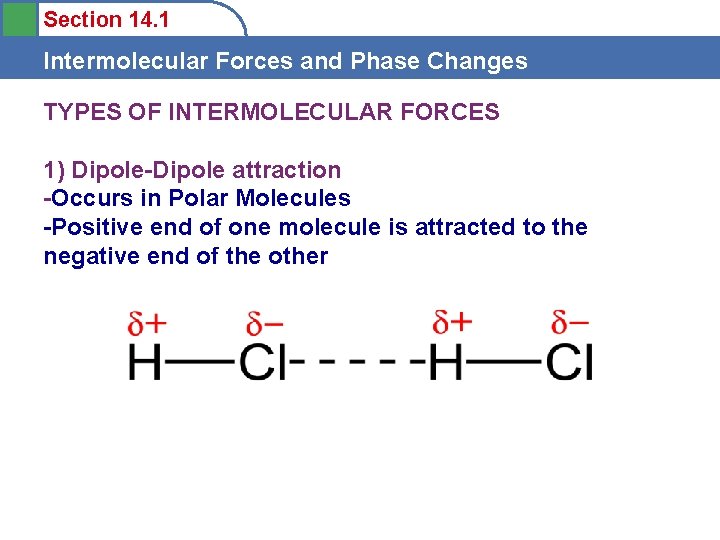
Section 14. 1 Intermolecular Forces and Phase Changes TYPES OF INTERMOLECULAR FORCES 1) Dipole-Dipole attraction -Occurs in Polar Molecules -Positive end of one molecule is attracted to the negative end of the other

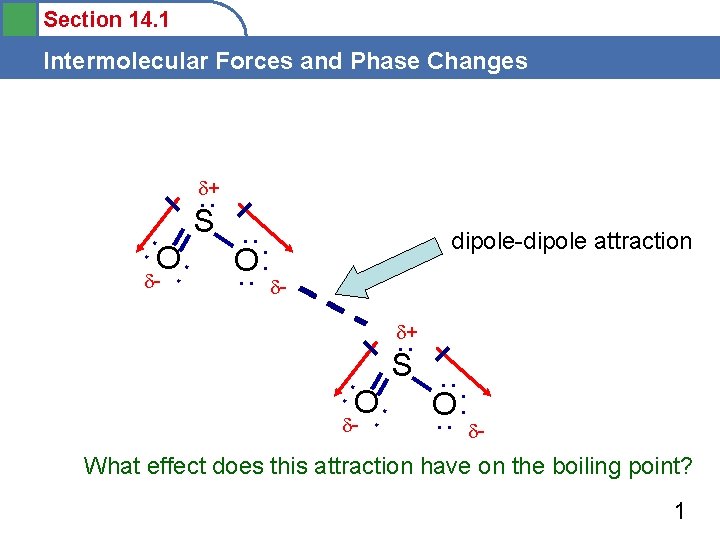
Section 14. 1 Intermolecular Forces and Phase Changes + . . S. . : O O: : . . - dipole-dipole attraction + . . S. . : O O: : . . - - What effect does this attraction have on the boiling point? 1

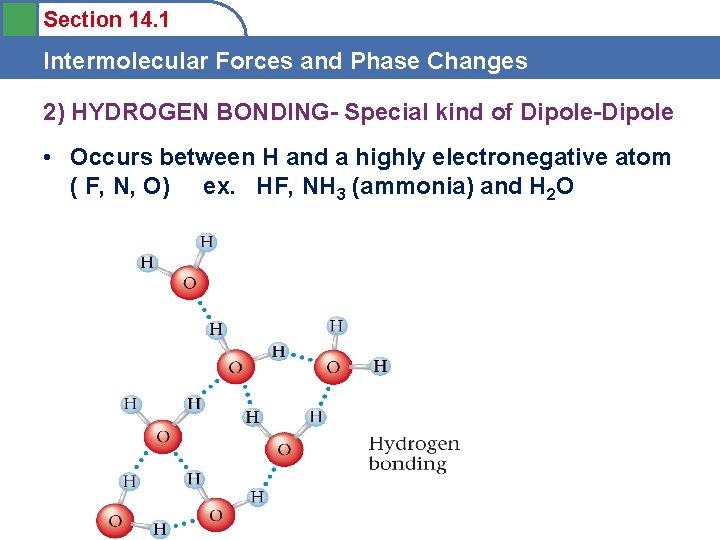
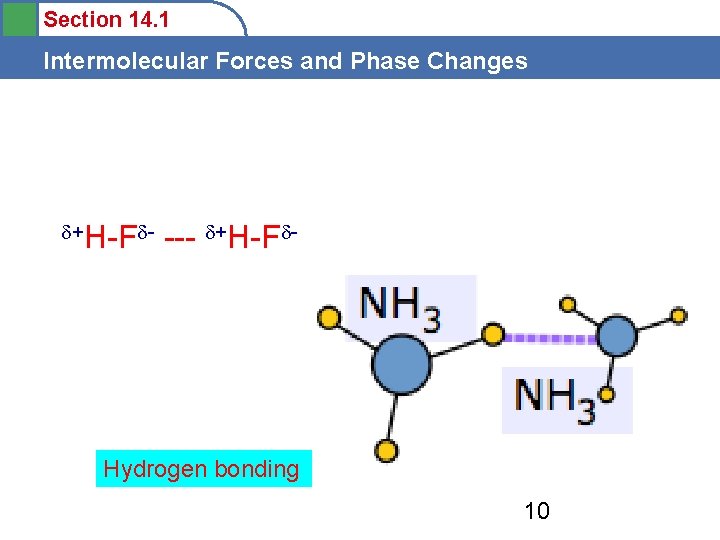
Section 14. 1 Intermolecular Forces and Phase Changes 2) HYDROGEN BONDING- Special kind of Dipole-Dipole • Occurs between H and a highly electronegative atom ( F, N, O) ex. HF, NH 3 (ammonia) and H 2 O

Section 14. 1 Intermolecular Forces and Phase Changes • H- bonding is a special kind of dipole –dipole that is much stronger. • Occurs in water and some other compounds and affects their physical properties.

Section 14. 1 Intermolecular Forces and Phase Changes +H-F - --- +H-F - Hydrogen bonding 10

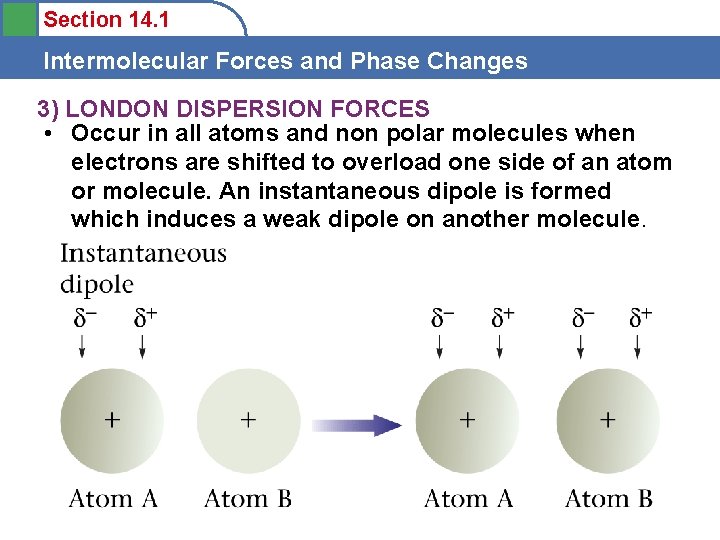
Section 14. 1 Intermolecular Forces and Phase Changes 3) LONDON DISPERSION FORCES • Occur in all atoms and non polar molecules when electrons are shifted to overload one side of an atom or molecule. An instantaneous dipole is formed which induces a weak dipole on another molecule.

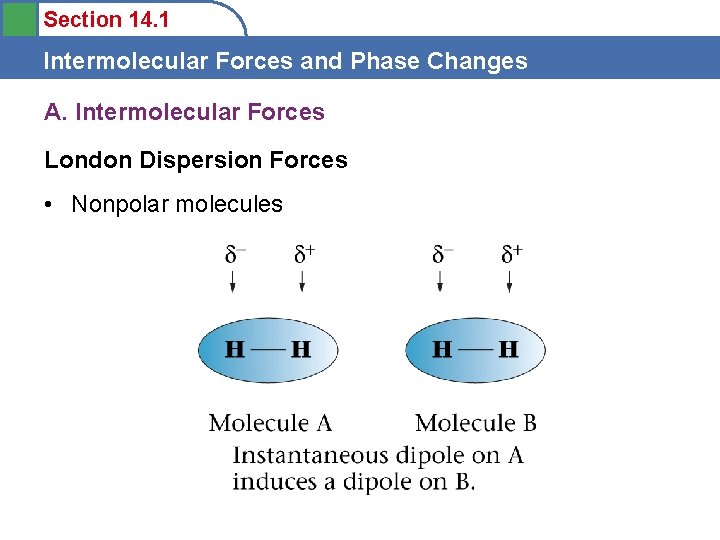
Section 14. 1 Intermolecular Forces and Phase Changes A. Intermolecular Forces London Dispersion Forces • Nonpolar molecules

Section 14. 1 Intermolecular Forces and Phase Changes • London Dispersion forces become stronger as the mass increases because heavier atoms have more electrons. Which of these do you expect to have stronger London Dispersion Forces? All of them are non-polar a) Kr or Ne b) CF 4 and CH 4 c) Br 2 or I 2

Section 14. 1 Intermolecular Forces and Phase Changes Intermolecular forces and Physical properties In general, the stronger the IMF, the more the energy required to break the bond. The strongest IMF is H-bond, followed by D-D and weakest are L-D.

Section 14. 1 Intermolecular Forces and Phase Changes IMFs and Physical properties Inter molecular forces affect many physical properties of substances. Some of them are a) Melting and Boiling point: Melting and Boiling points generally increase when you go from top to bottom on the periodic table but polar substances have high melting and boiling points. Non-polar substances have lower melting and boiling points. Substances that have H-bonding will have even higher melting and boiling points as compared to regular dipole-dipole.

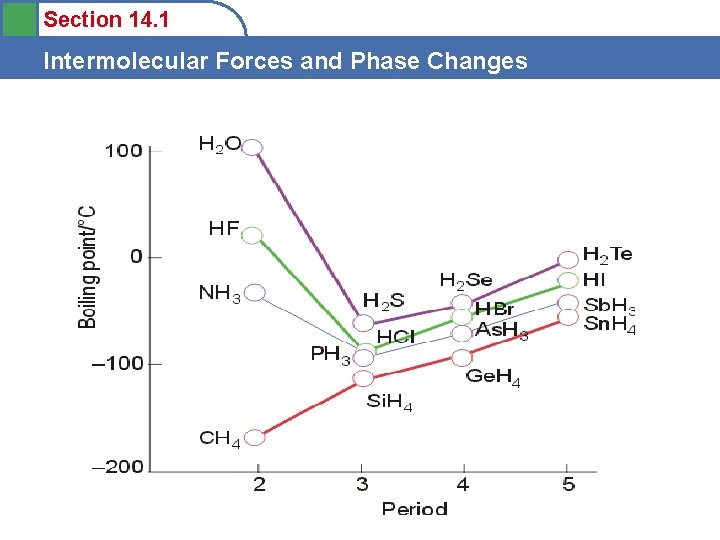
Section 14. 1 Intermolecular Forces and Phase Changes • The following graph shows the boiling points of some substances. NH 3, H 2 O and HF all have H-bonds while CH 4 doesn’t. That’s why CH 4 follows the regular pattern, while the other 3 don’t.

Section 14. 1 Intermolecular Forces and Phase Changes

Section 14. 1 Intermolecular Forces and Phase Changes b) Surface tension- Polar molecules have high surface tension because the adjacent molecules stick to each other forming a film or layer on the surface.

Section 14. 1 Intermolecular Forces and Phase Changes • c) Solubility. Define Solute- The substance being dissolved in a solution (ex. Salt) Define Solvent- The dissolving medium in a solution (ex. water) Define Solution- A homogeneous mixture of a solute and solvent (ex. Salt-water solution) Polar substances dissolve in other polar substances, while non polar substances dissolve in other non-polar substances. “Like dissolves like”.

Section 14. 1 Intermolecular Forces and Phase Changes • Ionic substances also dissolve in polar solvents for example salt (Na. Cl also dissolves in water through a special kind of force called ion-dipole