Section 14 1 Intermolecular Forces and Phase Changes




























- Slides: 60

Section 14. 1 Intermolecular Forces and Phase Changes Objectives 1. To learn about hydrogen bonding, dipole-dipole, and London dispersion forces (van der Waals) 2. To understand the effect of intermolecular forces on the properties of liquids 3. To learn about interactions among water molecules 4. To predict relative physical properties based on intermolecular forces of attraction

Section 14. 1 Intermolecular Forces and Phase Changes A. Intermolecular Forces Reviewing what we know Gases Solids • Low density • Highly compressible • Fill container • High density • Slightly compressible • Rigid (keeps its shape)

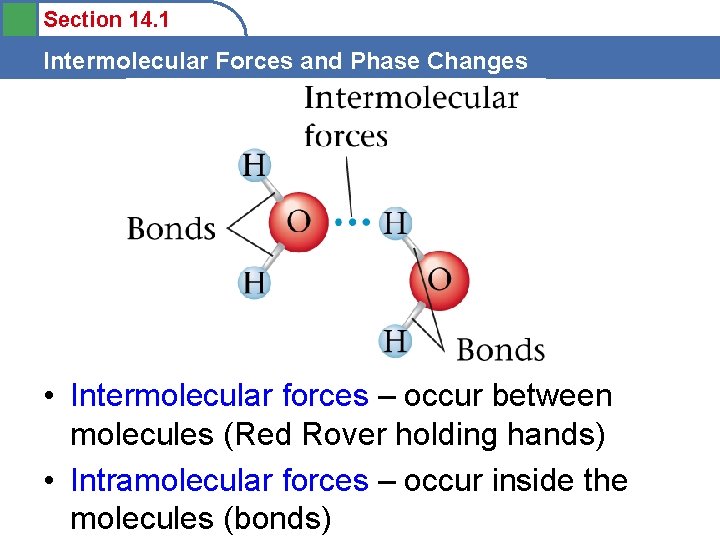
Section 14. 1 Intermolecular Forces and Phase Changes • Intermolecular forces – occur between molecules (Red Rover holding hands) • Intramolecular forces – occur inside the molecules (bonds)

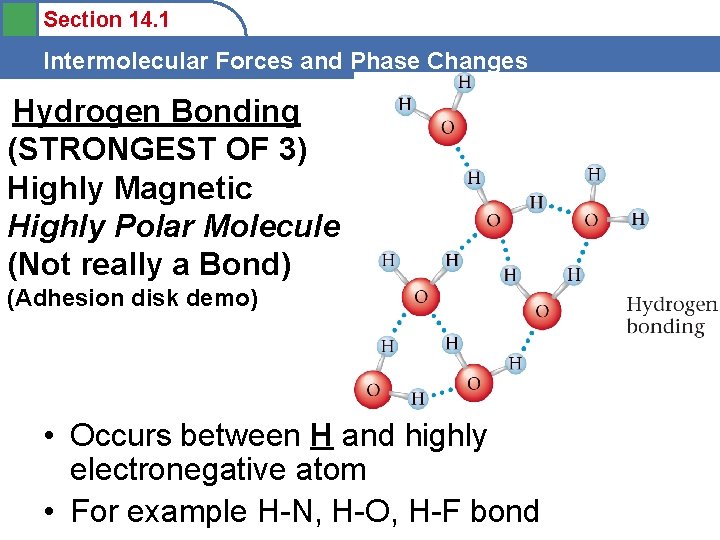
Section 14. 1 Intermolecular Forces and Phase Changes Hydrogen Bonding (STRONGEST OF 3) Highly Magnetic Highly Polar Molecule (Not really a Bond) (Adhesion disk demo) • Occurs between H and highly electronegative atom • For example H-N, H-O, H-F bond

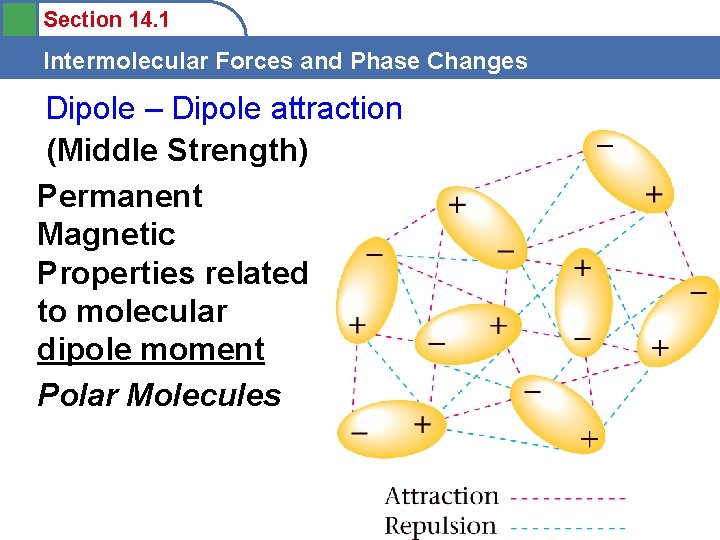
Section 14. 1 Intermolecular Forces and Phase Changes Dipole – Dipole attraction (Middle Strength) Permanent Magnetic Properties related to molecular dipole moment Polar Molecules

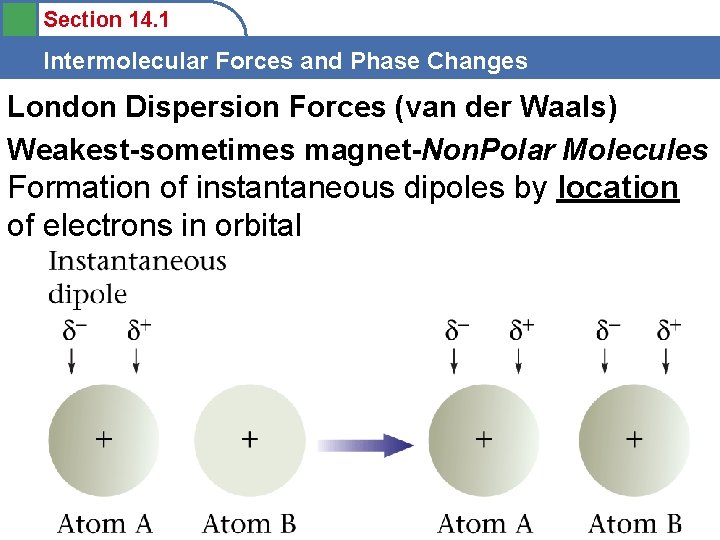
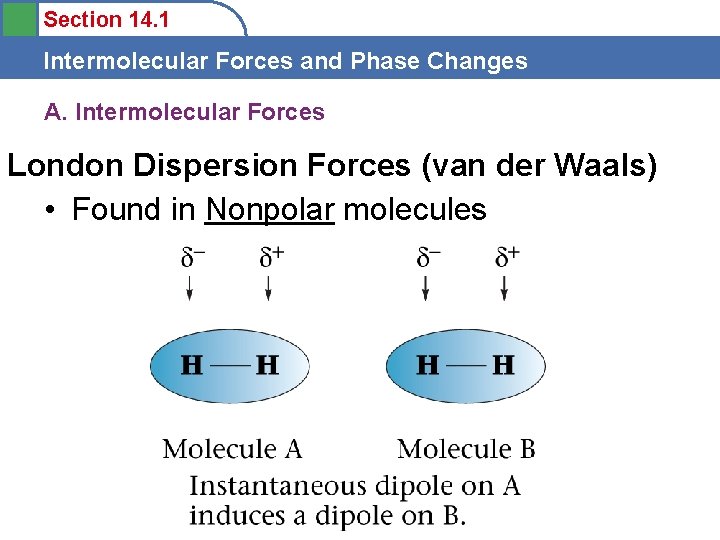
Section 14. 1 Intermolecular Forces and Phase Changes London Dispersion Forces (van der Waals) Weakest-sometimes magnet-Non. Polar Molecules Formation of instantaneous dipoles by location of electrons in orbital

Section 14. 1 Intermolecular Forces and Phase Changes A. Intermolecular Forces London Dispersion Forces (van der Waals) • Found in Nonpolar molecules

Section 14. 1 Intermolecular Forces and Phase Changes A. Intermolecular Forces • The nature of intermolecular forces is the same as the nature of intramolecular forces (bonds) – only to a varying degree. • Intermolecular forces of attraction are a weaker part of the continuum of magnetic attractive forces that include chemical bonding. • Weaker (Inter) (Intra) Stronger • ___________________ • VDW D-D H-Bond Covalent Polar Ionic

Section 14. 1 Intermolecular Forces and Phase Changes A. Intermolecular Forces • H bonding molecules will exhibit all 3 intermolecular forces of attraction • H Bonding forces will be the dominate • D-D molecules will exhibit D-D and vd. W • vd. W only vd. W

Section 14. 1 Intermolecular Forces and Phase Changes A. Summary of IMF Forces • • • VDW sometimes Magnet Non Polar Weak IMF Dipole – Dipole H-Bonding

Section 14. 1 Intermolecular Forces and Phase Changes • Draw L-D and predict polarity to determine which type of IMF will dominate. • NH 3 • HCl • BCl 3 • CH 3 Cl • CH 4 • H 2 O • H 2 S • HF

Section 14. 1 Intermolecular Forces and Phase Changes A. Intermolecular Forces London Dispersion Forces • Become stronger as the sizes of atoms or molecules increase ---WHY? • As atomic size increases, what else increases in number? • How would this affect the ability of the molecules to pull together? FP?

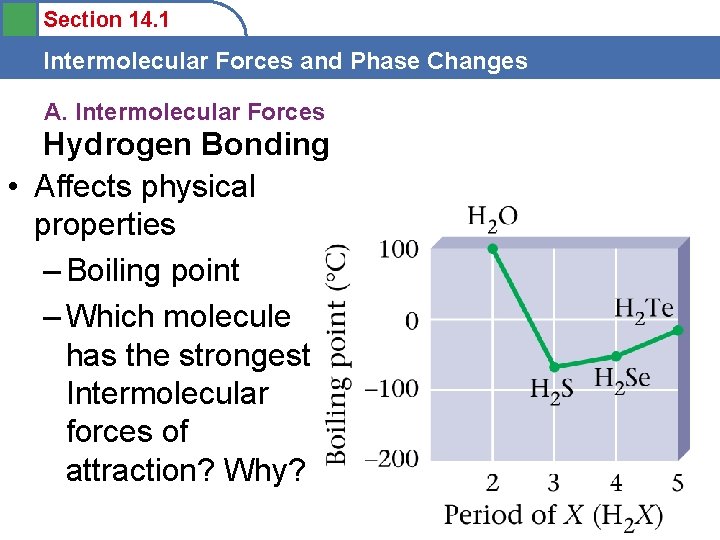
Section 14. 1 Intermolecular Forces and Phase Changes A. Intermolecular Forces Hydrogen Bonding • Affects physical properties – Boiling point – Which molecule has the strongest Intermolecular forces of attraction? Why?

Section 14. 1 Intermolecular Forces and Phase Changes A. Intermolecular Forces • Pour CH 3 OH vs H 2 O on tabletop • The strength of intermolecular forces of attraction and the ability of the molecules to hold together directly affect the temperature at which substances melt (or freeze) and boil (or condense). • See the Heating Curve…Explain on a molecular level what happens during the solid/liquid and liquid/gas phase change…. .

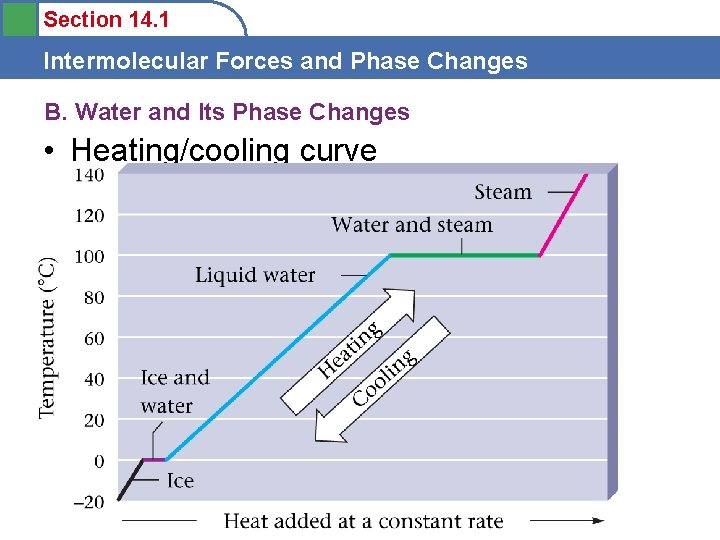
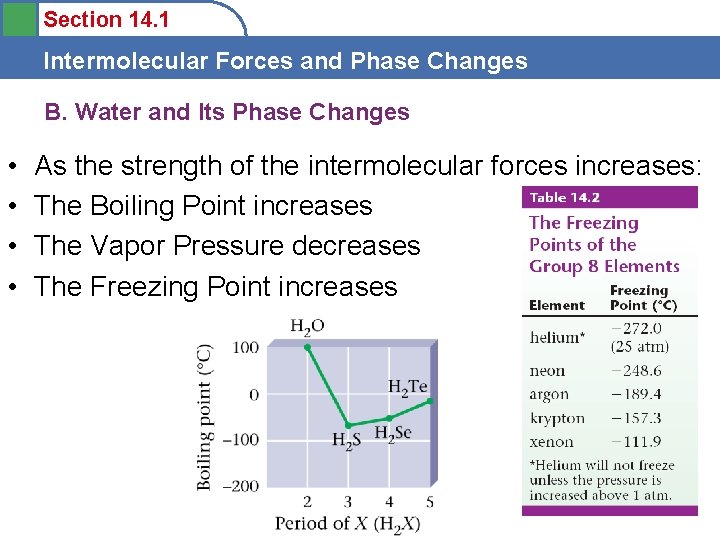
Section 14. 1 Intermolecular Forces and Phase Changes B. Water and Its Phase Changes • Heating/cooling curve

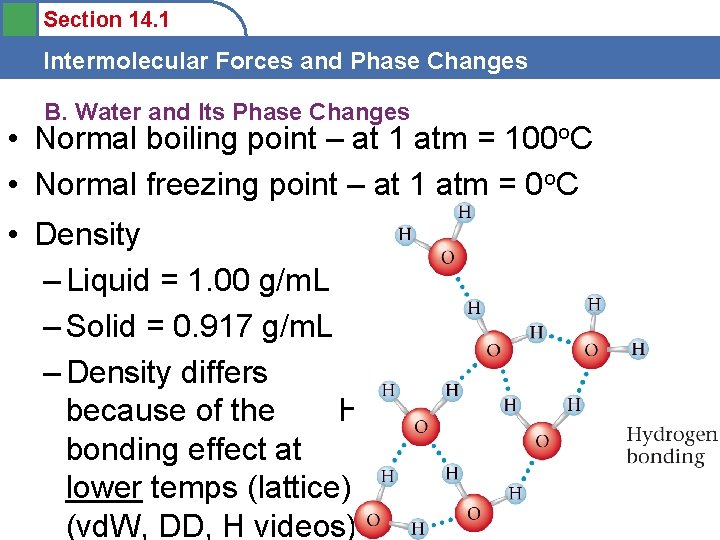
Section 14. 1 Intermolecular Forces and Phase Changes B. Water and Its Phase Changes • Normal boiling point – at 1 atm = 100 o. C • Normal freezing point – at 1 atm = 0 o. C • Density – Liquid = 1. 00 g/m. L – Solid = 0. 917 g/m. L – Density differs because of the Hbonding effect at lower temps (lattice) (vd. W, DD, H videos)

Section 14. 1 Intermolecular Forces and Phase Changes B. Water and Its Phase Changes • Let’s consider what happens on a molecular level when a substance changes phase from solid to liquid and liquid to gas. • Motion, space between molecules, forces to overcome? • Changes of state video • Thoughts on boiling water at room temperature? Thoughts only!!

Section 14. 1 Intermolecular Forces and Phase Changes B. Water and Its Phase Changes • • As the strength of the intermolecular forces increases: The Boiling Point increases The Vapor Pressure decreases The Freezing Point increases

Section 14. 1 Intermolecular Forces and Phase Changes B. Predicting Relative Physical Properties • Which of the following substances would have the higher boiling point? (Think polarity) • NH 3 or BCl 3 • CH 4 or CH 3 Cl • H 2 O or H 2 S • HF or HCl • Which would have the highest freezing point? Vapor Pressure?

Section 14. 1 Intermolecular Forces and Phase Changes B. Predicting Relative Physical Properties

Section 14. 1 Intermolecular Forces and Phase Changes

Section 14. 1 Intermolecular Forces and Phase Changes

Section 14. 1 Intermolecular Forces and Phase Changes Objectives Review 1. To learn about hydrogen bonding, dipole, and London dispersion forces (van der Waals) 2. To understand the effect of intermolecular forces on the properties of liquids 3. To learn about interactions among water molecules 4. To predict relative physical properties based on intermolecular forces of attraction 5. Work Session: Page 497 Review # 1 -5

Section 14. 1 Intermolecular Forces and Phase Changes Objectives • To understand use heat of fusion and heat of vaporization • To derive formulas • To use the 5 Step Method to find solutions using multiple equations

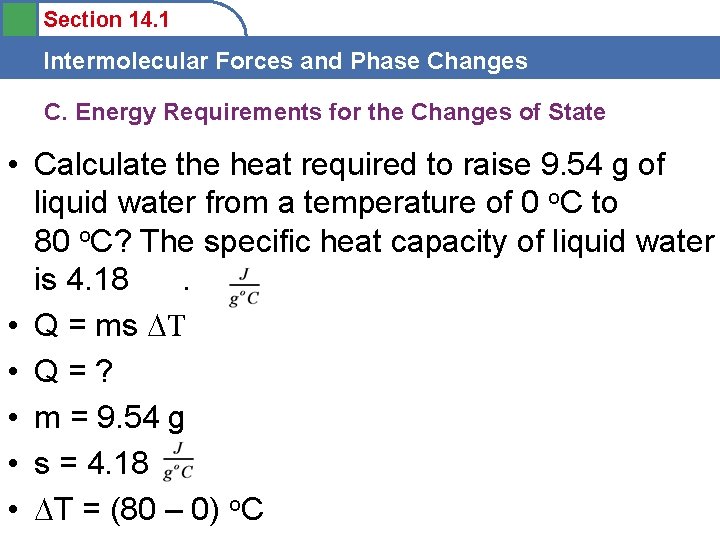
Section 14. 1 Intermolecular Forces and Phase Changes C. Energy Requirements for the Changes of State • Calculate the heat required to raise 9. 54 g of liquid water from a temperature of 0 o. C to 80 o. C? The specific heat capacity of liquid water is 4. 18. • Q = ms T • Q=? • m = 9. 54 g • s = 4. 18 • T = (80 – 0) o. C

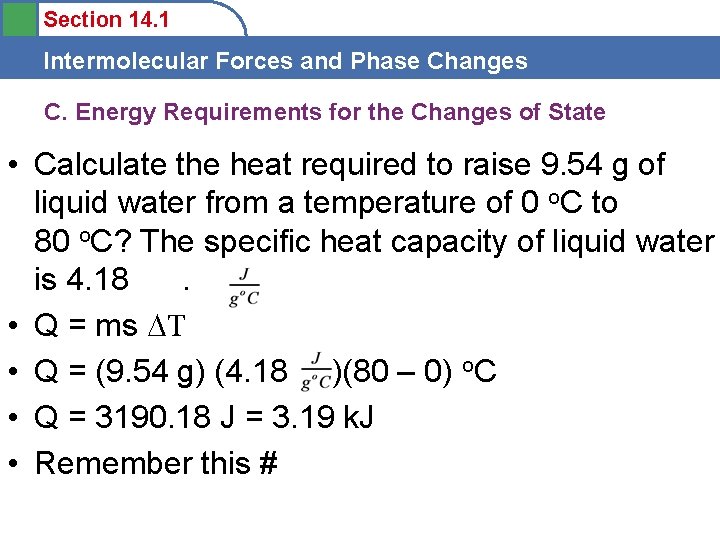
Section 14. 1 Intermolecular Forces and Phase Changes C. Energy Requirements for the Changes of State • Calculate the heat required to raise 9. 54 g of liquid water from a temperature of 0 o. C to 80 o. C? The specific heat capacity of liquid water is 4. 18. • Q = ms T • Q = (9. 54 g) (4. 18 )(80 – 0) o. C • Q = 3190. 18 J = 3. 19 k. J • Remember this #

Section 14. 1 Intermolecular Forces and Phase Changes C. Energy Requirements for the Changes of State • Changes of state are physical changes – No chemical bonds are broken

Section 14. 1 Intermolecular Forces and Phase Changes C. Energy Requirements for the Changes of State • Molar heat of fusion – energy required to melt (S-L) 1 mol of a substance • Molar heat of vaporization – energy required to change 1 mol of a liquid to its vapor …units of both? ?

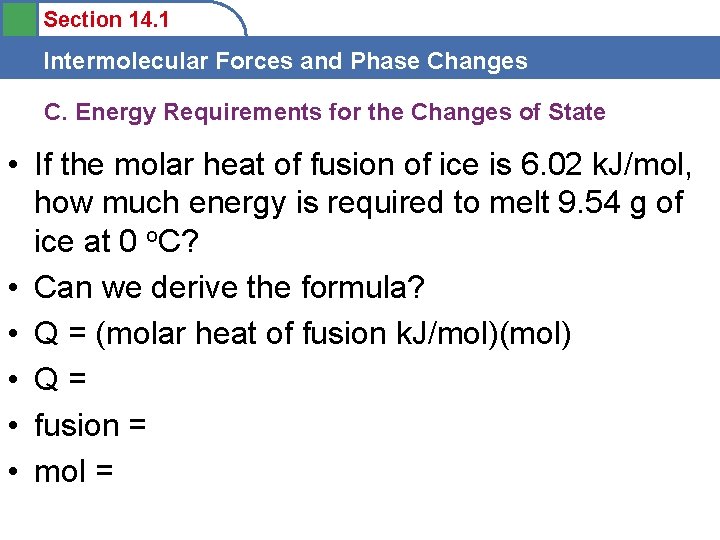
Section 14. 1 Intermolecular Forces and Phase Changes C. Energy Requirements for the Changes of State • If the molar heat of fusion of ice is 6. 02 k. J/mol, how much energy is required to melt 9. 54 g of ice at 0 o. C? • Can we derive the formula? • Q = (molar heat of fusion k. J/mol)(mol) • Q= • fusion = • mol =

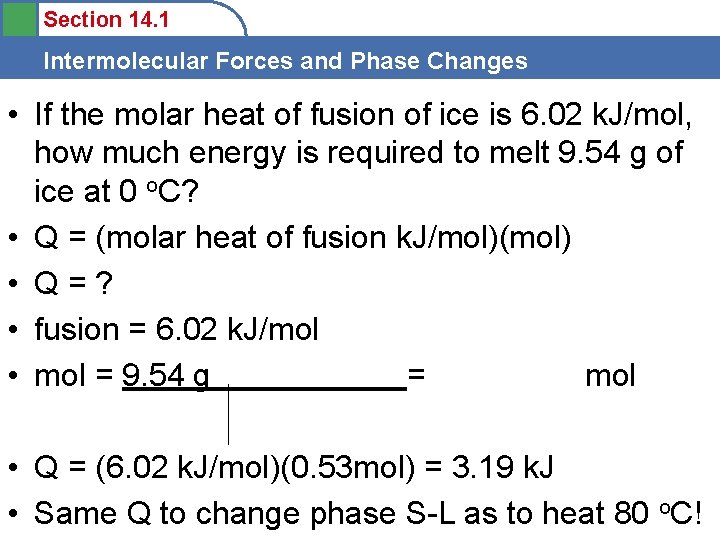
Section 14. 1 Intermolecular Forces and Phase Changes • If the molar heat of fusion of ice is 6. 02 k. J/mol, how much energy is required to melt 9. 54 g of ice at 0 o. C? • Q = (molar heat of fusion k. J/mol)(mol) • Q=? • fusion = 6. 02 k. J/mol • mol = 9. 54 g = mol • Q = (6. 02 k. J/mol)(0. 53 mol) = 3. 19 k. J • Same Q to change phase S-L as to heat 80 o. C!

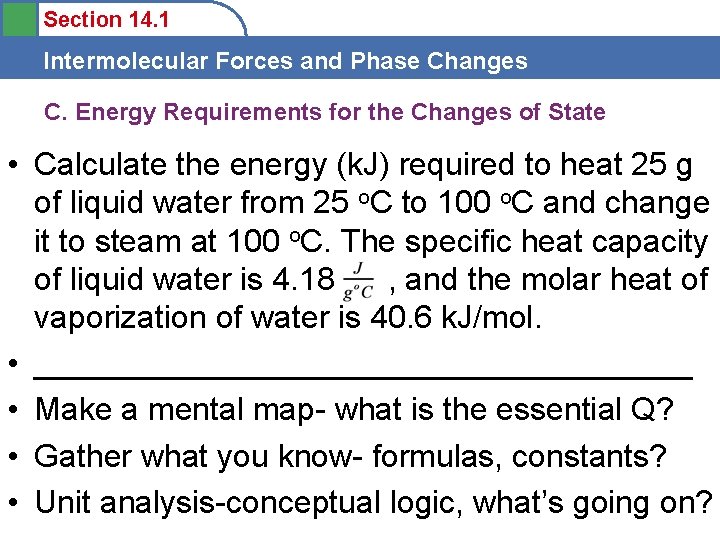
Section 14. 1 Intermolecular Forces and Phase Changes C. Energy Requirements for the Changes of State • Calculate the energy (k. J) required to heat 25 g of liquid water from 25 o. C to 100 o. C and change it to steam at 100 o. C. The specific heat capacity of liquid water is 4. 18 , and the molar heat of vaporization of water is 40. 6 k. J/mol. • ___________________ • Make a mental map- what is the essential Q? • Gather what you know- formulas, constants? • Unit analysis-conceptual logic, what’s going on?

Section 14. 1 Intermolecular Forces and Phase Changes C. Energy Requirements for the Changes of State • Calculate the energy (k. J) required to heat 25 g of liquid water from 25 o. C to 100 o. C and change it to steam at 100 o. C. The specific heat capacity of liquid water is 4. 18 , and the molar heat of vaporization of water is 40. 6 k. J/mol. • Energy in k. J to take Liq from 25 o. C to steam at 100 o. C • 1) Warm w/o phase change 2) phase change • Two formulas, two thermodynamic properties

Section 14. 1 Intermolecular Forces and Phase Changes C. Energy Requirements for the Changes of State • Calculate the energy (k. J) required to heat 25 g of liquid water from 25 o. C to 100 o. C and change it to steam at 100 o. C. The specific heat capacity of liquid water is 4. 18 , and the molar heat of vaporization of water is 40. 6 k. J/mol. • 1) Warm: Q = ms T • Q= • m= • s= • T =

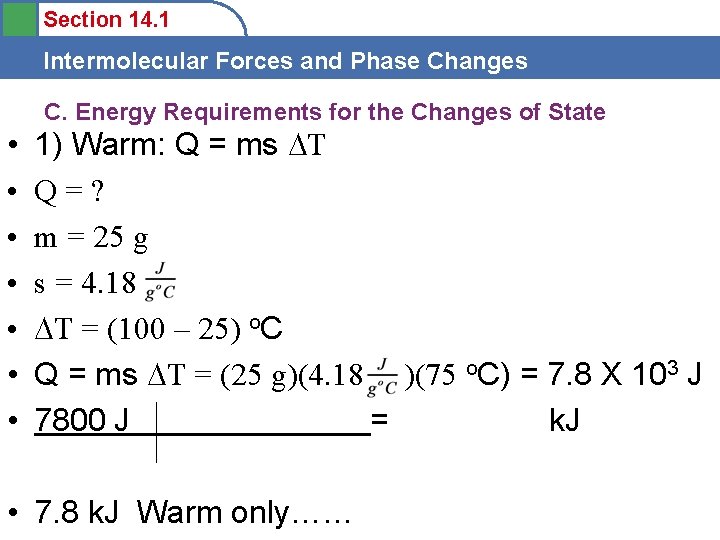
Section 14. 1 Intermolecular Forces and Phase Changes C. Energy Requirements for the Changes of State • • 1) Warm: Q = ms T Q=? m = 25 g s = 4. 18 T = (100 – 25) o. C Q = ms T = (25 g)(4. 18 )(75 o. C) = 7. 8 X 103 J 7800 J = k. J • 7. 8 k. J Warm only……

Section 14. 1 Intermolecular Forces and Phase Changes C. Energy Requirements for the Changes of State • Calculate the energy (k. J) required to heat 25 g of liquid water from 25 o. C to 100 o. C and change it to steam at 100 o. C. The specific heat capacity of liquid water is 4. 18 , and the molar heat of vaporization of water is 40. 6 k. J/mol. • 2) Phase change • Q = (molar heat of vap k. J/mol)(mol) • Q=? • vap = 40. 6 k. J/mol • mol = 25 g = mol • Q = (40. 6 k. J/mol)(1. 39 mol) = 56. 43 k. J

Section 14. 1 Intermolecular Forces and Phase Changes C. Energy Requirements for the Changes of State • Calculate the energy (k. J) required to heat 25 g of liquid water from 25 o. C to 100 o. C and change it to steam at 100 o. C. The specific heat capacity of liquid water is 4. 18 , and the molar heat of vaporization of water is 40. 6 k. J/mol. • 1) Warm = 7. 8 k. J • 2) Phase change = 56. 43 k. J • Total --------- = 64. 23 k. J • Notice that most of the energy is required for the phase change event.

Section 14. 1 Intermolecular Forces and Phase Changes C. Energy Requirements for the Changes of State • molar heat of vaporization of water is 40. 6 k. J/mol • molar heat of fusion of water is 6. 02 k. J/mol • Does it take more energy to melt ice or boil liquid? Why?

Section 14. 1 Intermolecular Forces and Phase Changes Objectives Review • To understand use heat of fusion and heat of vaporization • To derive formulas • To use the 5 Step Method to find solutions using multiple equations • Work Session: Page 497 #6 -7 Practice Problem 14. 2

Section 14. 2 Vapor Pressure and Boiling Point Objectives 1. To understand the relationship among vaporization, condensation and vapor pressure 2. To relate the boiling point of water to its vapor pressure 3. To predict relative physical properties based on intermolecular forces of attraction

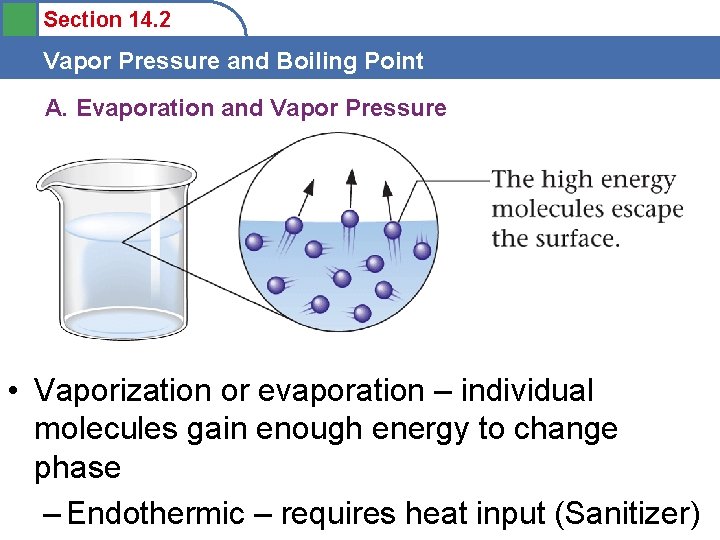
Section 14. 2 Vapor Pressure and Boiling Point A. Evaporation and Vapor Pressure • Vaporization or evaporation – individual molecules gain enough energy to change phase – Endothermic – requires heat input (Sanitizer)

Section 14. 2 Vapor Pressure and Boiling Point A. Evaporation and Vapor Pressure • Amount of liquid first decreases then becomes constant - H 2 O bottle • Condensation - process by which vapor molecules convert to a liquid • When no further change is visible the opposing processes balance each other equilibrium

Section 14. 2 Vapor Pressure and Boiling Point A. Evaporation and Vapor Pressure • Vapor pressure - pressure of the vapor present at equilibrium with its liquid Vapor pressures vary widely relates to intermolecular forces

Section 14. 2 Vapor Pressure and Boiling Point A. Evaporation and Vapor Pressure 1. Vapor pressure - pressure of the vapor present at equilibrium with its liquidrelates to intermolecular forces 2. Molecules with higher intermolecular forces of attraction will have ____ vapor pressures because _____________. • lower, molecules will hold together more.

Section 14. 2 Vapor Pressure and Boiling Point A. Evaporation and Vapor Pressure • Which of the following substances would have the higher vapor pressure? Why? • NH 3 or BF 3 • CH 4 or CH 2 Cl 2 • H 2 O or CH 3 OH (methyl alcohol) • CH 3 OH or CH 3 CH 2 CH 2 OH (butyl alc) What is the BP of methyl alcohol? Butyl alc?

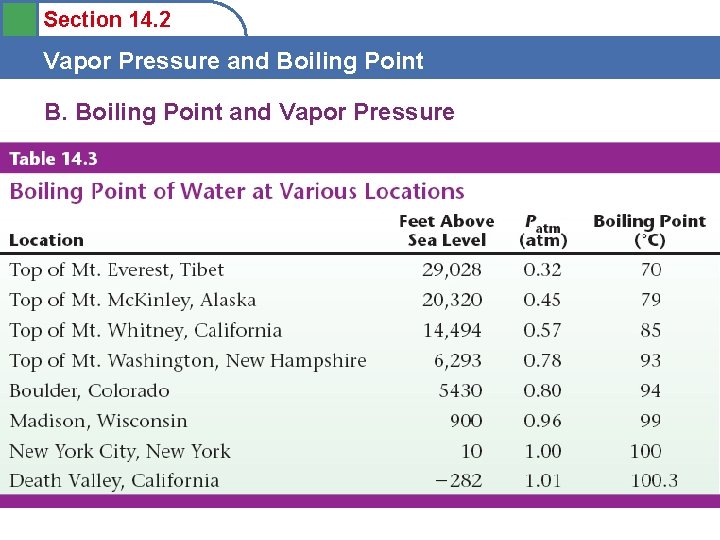
Section 14. 2 Vapor Pressure and Boiling Point B. Boiling Point and Vapor Pressure

Section 14. 2 Vapor Pressure and Boiling Point B. Boiling Point and Vapor Pressure

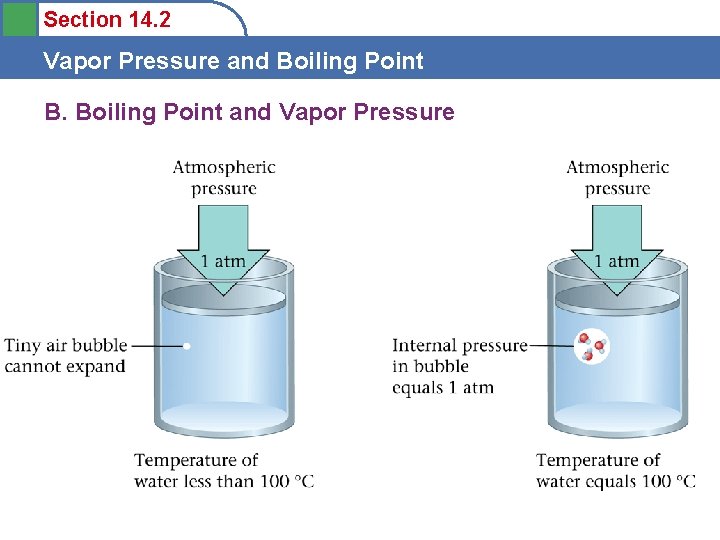
Section 14. 2 Vapor Pressure and Boiling Point B. Boiling Point and Vapor Pressure • Boiling point is defined as occurring when the vapor pressure of the liquid is equal to the total external pressure (atmospheric pressure). Not defined by temperature! • Boil water at room temp? ? • Boil water with ice?

Section 14. 2 Vapor Pressure and Boiling Point B. Boiling Point and Vapor Pressure • Which of the following substances would have the higher……BP FP VP • Ge. H 4 or As. H 3 • CCl 4 or H 2 Te • CO 2 or H 2 S • HF or HBr • The amazing floating steel!

Section 14. 2 Vapor Pressure and Boiling Point Objectives Review 1. To understand the relationship among vaporization, condensation and vapor pressure 2. To relate the boiling point of water to its vapor pressure 3. To predict relative physical properties based on IMF’s 4. To reiterate the difference between IMF and bonds 5. Work Session: Page 503 # 1, 2, 4, 5, 6

Section 14. 3 Properties of Solids Objectives 1. To learn about the types of crystalline solids 2. To understand the interparticle forces in crystalline solids 3. To learn how the bonding in metals determines metallic properties

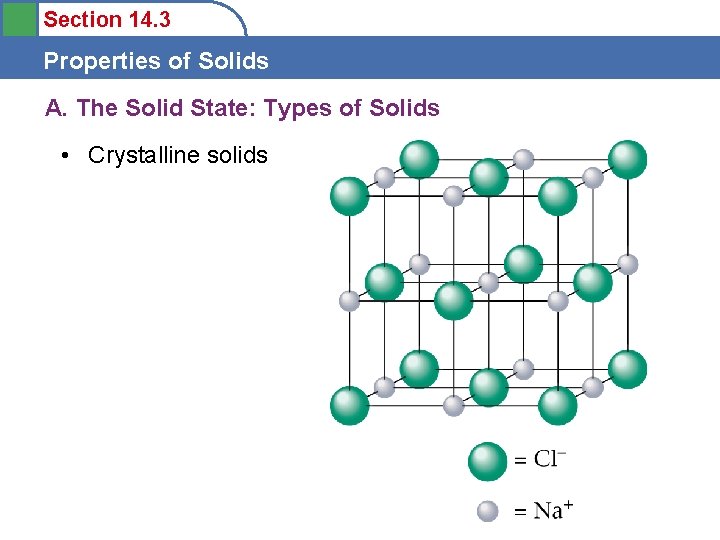
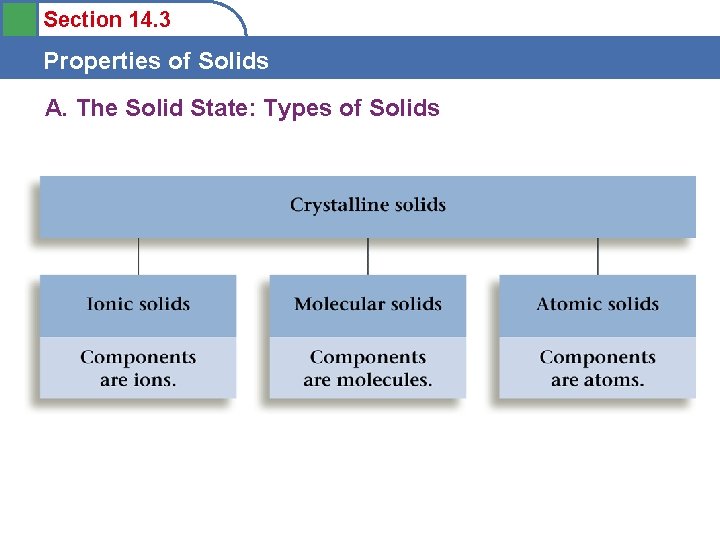
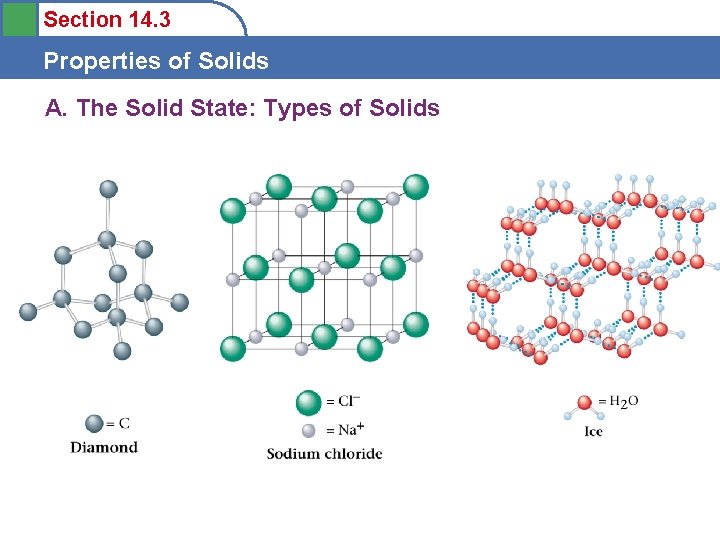
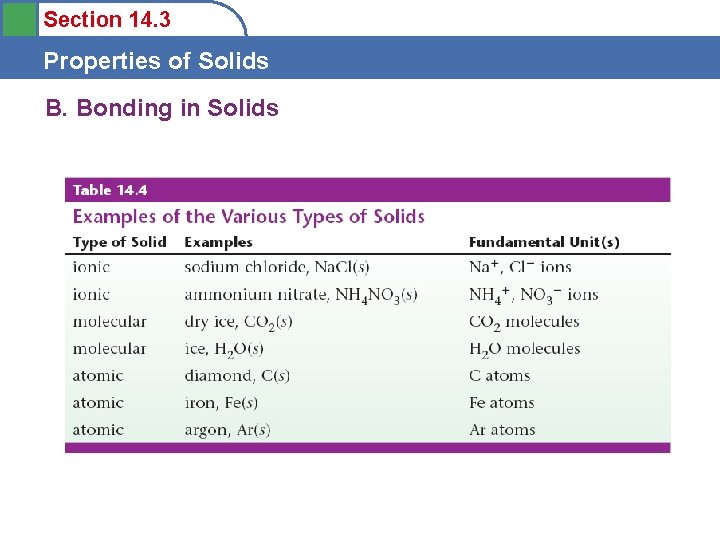
Section 14. 3 Properties of Solids A. The Solid State: Types of Solids • Crystalline solids

Section 14. 3 Properties of Solids A. The Solid State: Types of Solids

Section 14. 3 Properties of Solids A. The Solid State: Types of Solids

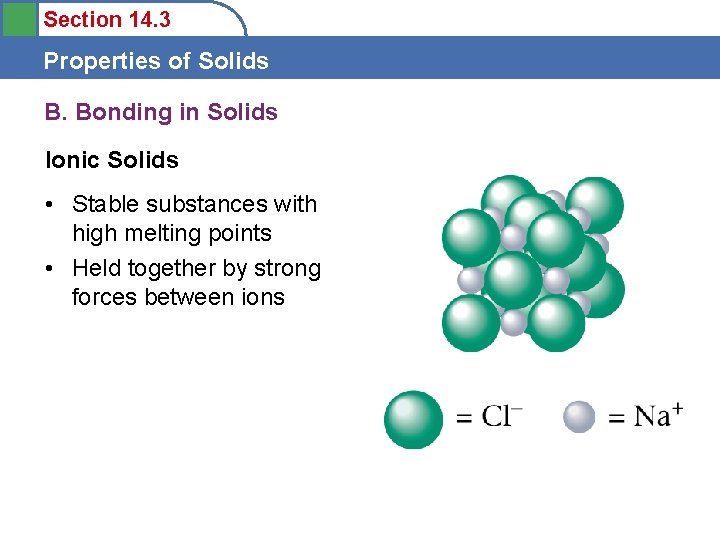
Section 14. 3 Properties of Solids B. Bonding in Solids

Section 14. 3 Properties of Solids B. Bonding in Solids Ionic Solids • Stable substances with high melting points • Held together by strong forces between ions

Section 14. 3 Properties of Solids B. Bonding in Solids Molecular Solids • Fundamental particle is a molecule • Melt at relatively low temperatures • Held together by weak intermolecular forces

Section 14. 3 Properties of Solids B. Bonding in Solids Atomic Solids • Fundamental particle is the atom • Properties vary greatly – Group 8 - low melting points – Diamond - very high melting point

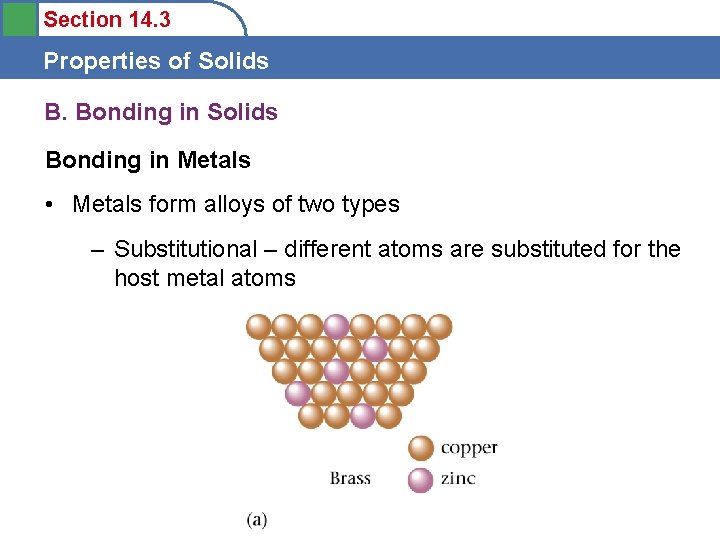
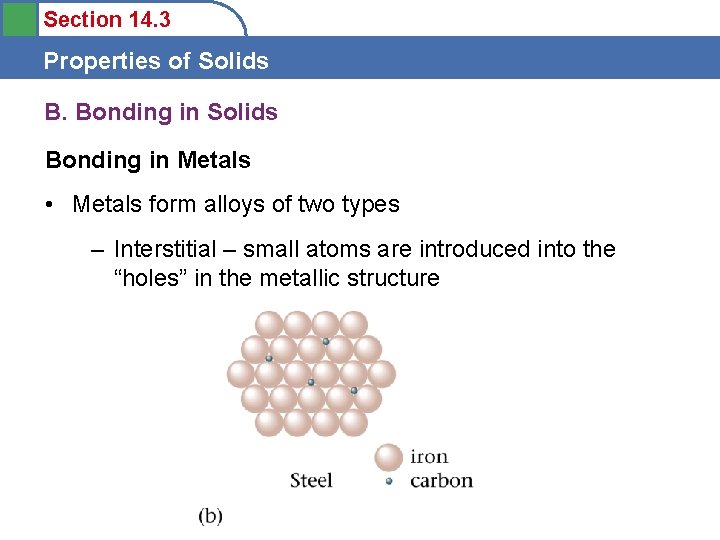
Section 14. 3 Properties of Solids B. Bonding in Solids Bonding in Metals • Metals are held together by nondirectional covalent bonds (called the electron sea model) among the closely packed atoms • Video clips?

Section 14. 3 Properties of Solids B. Bonding in Solids Bonding in Metals • Metals form alloys of two types – Substitutional – different atoms are substituted for the host metal atoms

Section 14. 3 Properties of Solids B. Bonding in Solids Bonding in Metals • Metals form alloys of two types – Interstitial – small atoms are introduced into the “holes” in the metallic structure