Section 12 4 Structure of Molecules Objectives 1
Section 12. 4 Structure of Molecules Objectives 1. To understand molecular structure and bond angles 2. To learn to predict molecular geometry from the number of electron pairs 3. To learn to apply the VSEPR model to molecules with double bonds
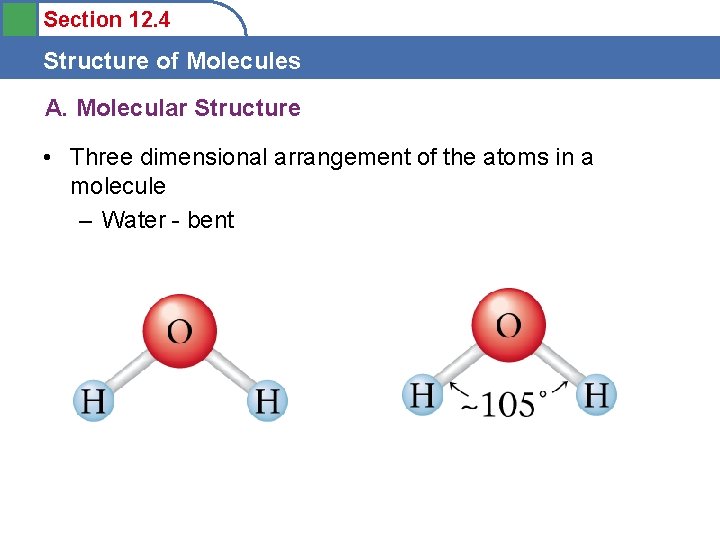
Section 12. 4 Structure of Molecules A. Molecular Structure • Three dimensional arrangement of the atoms in a molecule – Water - bent
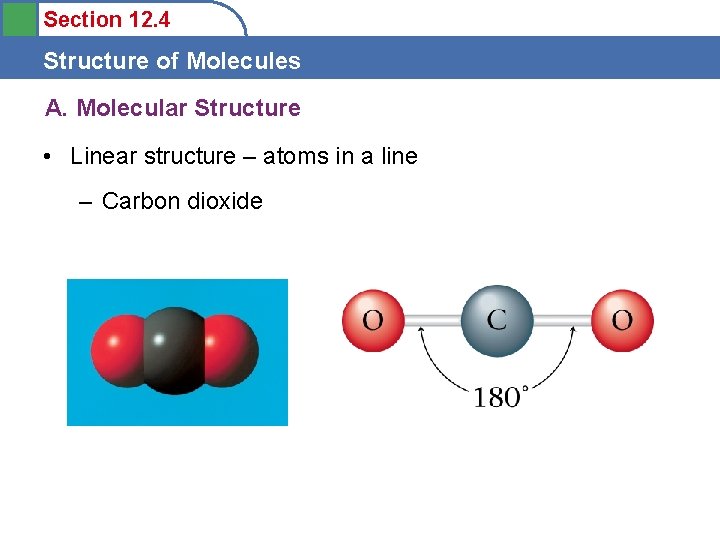
Section 12. 4 Structure of Molecules A. Molecular Structure • Linear structure – atoms in a line – Carbon dioxide
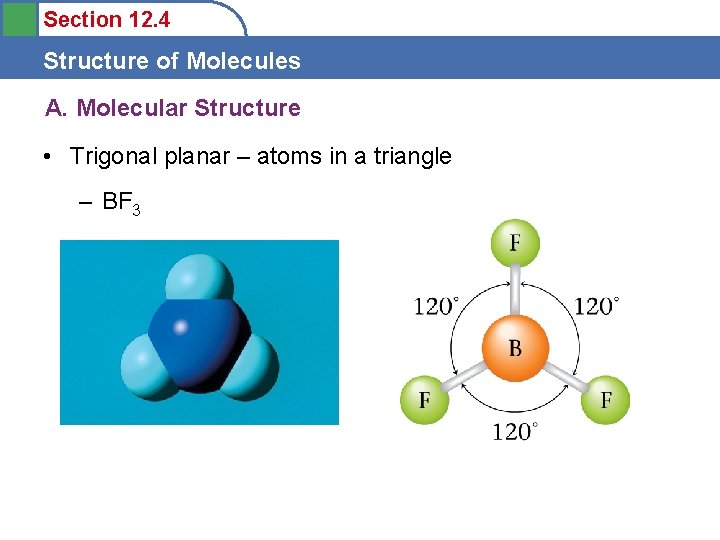
Section 12. 4 Structure of Molecules A. Molecular Structure • Trigonal planar – atoms in a triangle – BF 3
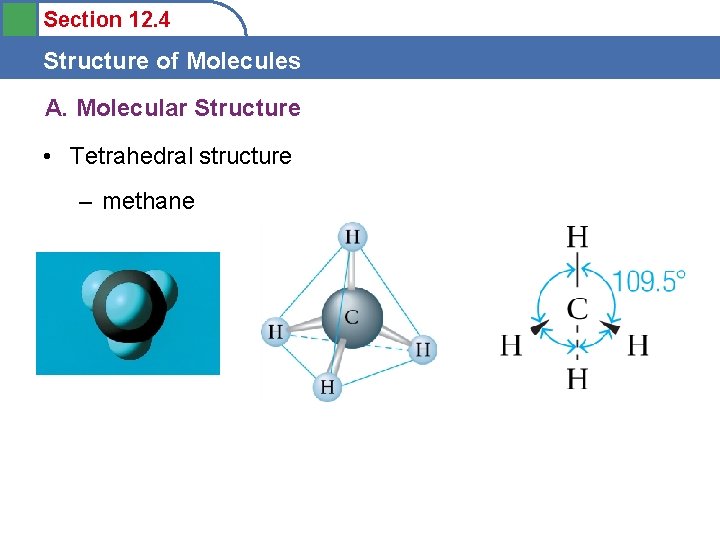
Section 12. 4 Structure of Molecules A. Molecular Structure • Tetrahedral structure – methane
Section 12. 4 Structure of Molecules B. The VSEPR Model • Valence shell electron pair repulsion (VSEPR) model – Molecular structure is determined by minimizing repulsions between electron pairs
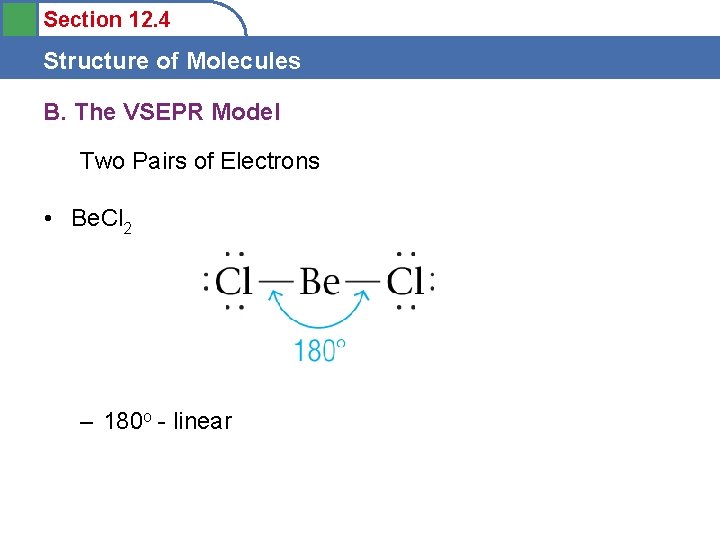
Section 12. 4 Structure of Molecules B. The VSEPR Model Two Pairs of Electrons • Be. Cl 2 – 180 o - linear
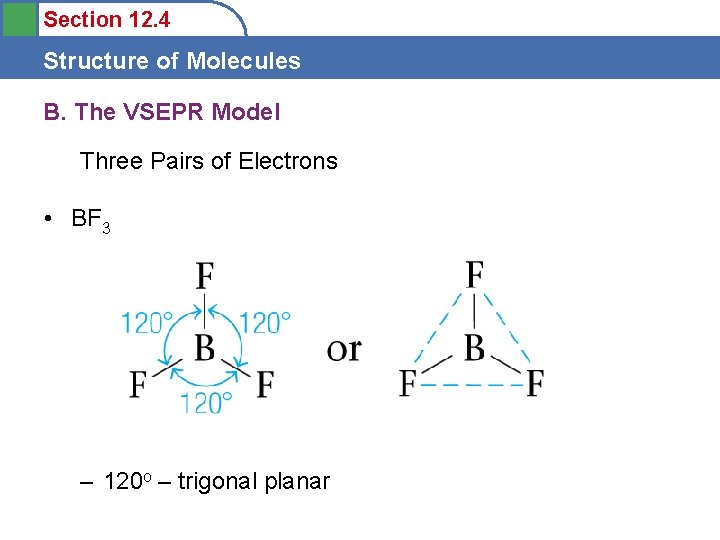
Section 12. 4 Structure of Molecules B. The VSEPR Model Three Pairs of Electrons • BF 3 – 120 o – trigonal planar
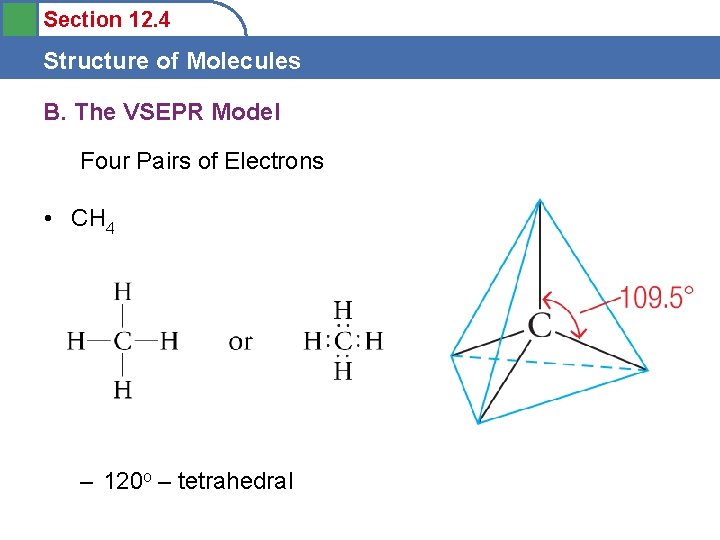
Section 12. 4 Structure of Molecules B. The VSEPR Model Four Pairs of Electrons • CH 4 – 120 o – tetrahedral
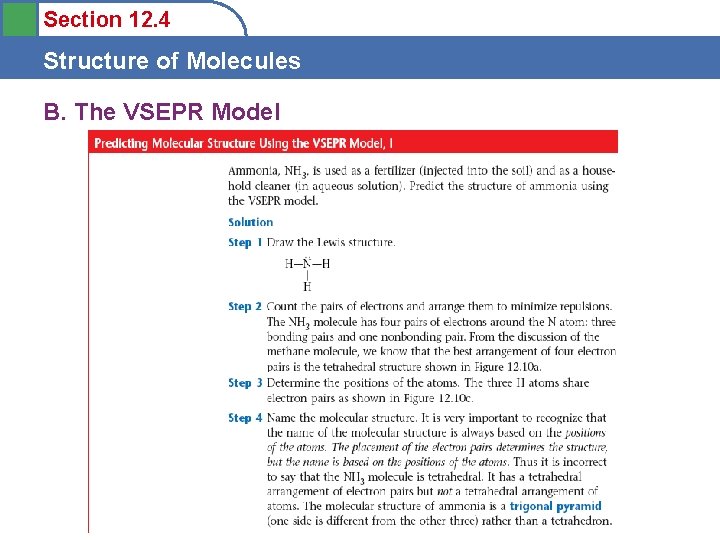
Section 12. 4 Structure of Molecules B. The VSEPR Model
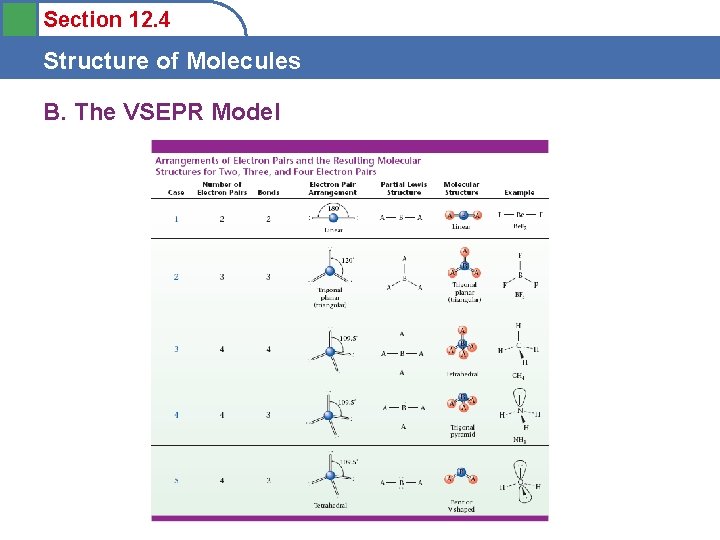
Section 12. 4 Structure of Molecules B. The VSEPR Model
Section 12. 4 Structure of Molecules C. Molecules with Double Bonds When using VSEPR model to predict molecular geometry of a molecule • a double bond is counted the same as a single electron pair
- Slides: 12