Section 10 5 Formulas of Hydrates Explain what









- Slides: 21

Section 10. 5 Formulas of Hydrates • Explain what a hydrate is and relate the name of the hydrate to its composition. • Determine the formula of a hydrate from laboratory data. Hydrates are solid ionic compounds in which water molecules are trapped.

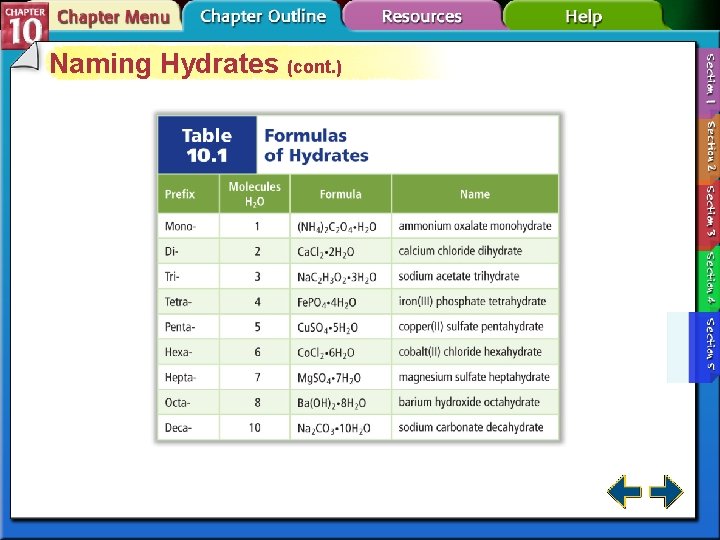
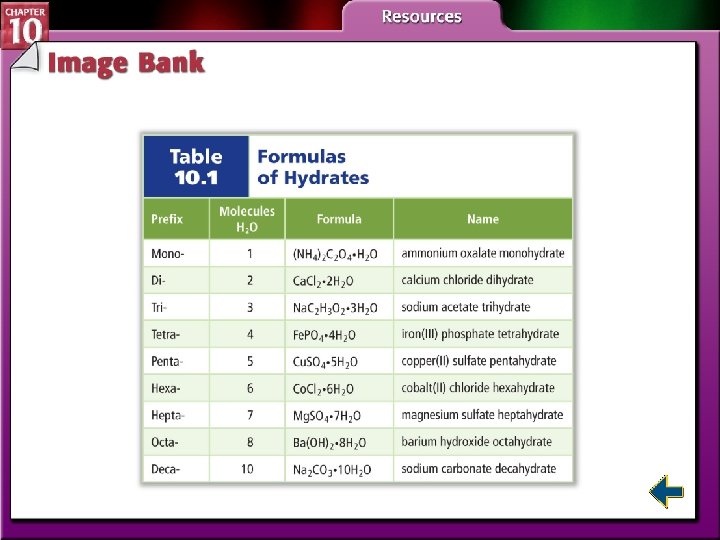
Naming Hydrates • A hydrate is a compound that has a specific number of water molecules bound to its atoms. • The number of water molecules associated with each formula unit of the compound is written following a dot. • Sodium carbonate decahydrate = Na 2 CO 3 • 10 H 2 O

Naming Hydrates (cont. )

Analyzing a Hydrate • When heated, water molecules are released from a hydrate leaving an anhydrous compound. • To determine the formula of a hydrate, find the number of moles of water associated with 1 mole of hydrate.

Analyzing a Hydrate (cont. ) • Weigh hydrate. • Heat to drive off the water. • Weigh the anhydrous compound. • Subtract and convert the difference to moles. • The ratio of moles of water to moles of anhydrous compound is the coefficient for water in the hydrate.

Use of Hydrates • Anhydrous forms of hydrates are often used to absorb water, particularly during shipment of electronic and optical equipment. • In chemistry labs, anhydrous forms of hydrates are used to remove moisture from the air and keep other substances dry.

Section 10. 5 Assessment Heating a hydrate causes what to happen? A. Water is driven from the hydrate. B. The hydrate melts. C. The hydrate conducts electricity. D. There is no change in the hydrate. A. B. C. D. A B C D

Section 10. 5 Assessment A hydrate that has been heated and the water driven off is called: A. dehydrated compound B. antihydrated compound C. anhydrous compound D. hydrous compound A. B. C. D. A B C D

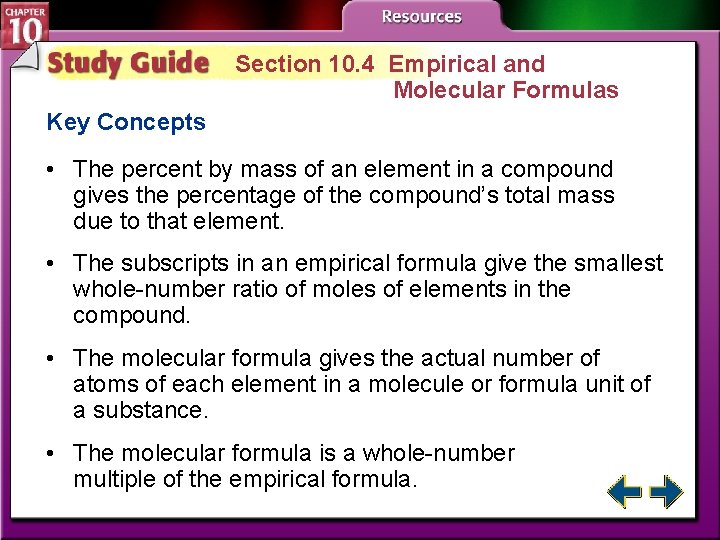
Section 10. 4 Empirical and Molecular Formulas Key Concepts • The percent by mass of an element in a compound gives the percentage of the compound’s total mass due to that element. • The subscripts in an empirical formula give the smallest whole-number ratio of moles of elements in the compound. • The molecular formula gives the actual number of atoms of each element in a molecule or formula unit of a substance. • The molecular formula is a whole-number multiple of the empirical formula.

Section 10. 5 Formulas of Hydrates Key Concepts • The formula of a hydrate consists of the formula of the ionic compound and the number of water molecules associated with one formula unit. • The name of a hydrate consists of the compound name and the word hydrate with a prefix indicating the number of water molecules in 1 mol of the compound. • Anhydrous compounds are formed when hydrates are heated.

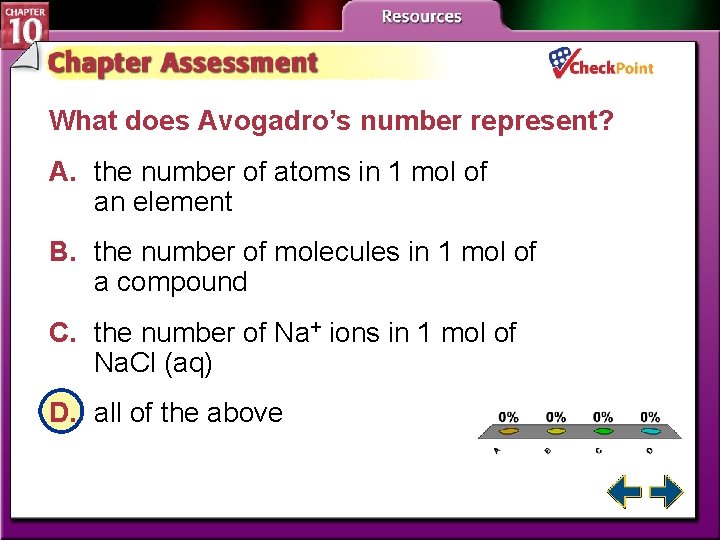
What does Avogadro’s number represent? A. the number of atoms in 1 mol of an element B. the number of molecules in 1 mol of a compound C. the number of Na+ ions in 1 mol of Na. Cl (aq) D. all of the above A. B. C. D. A B C D

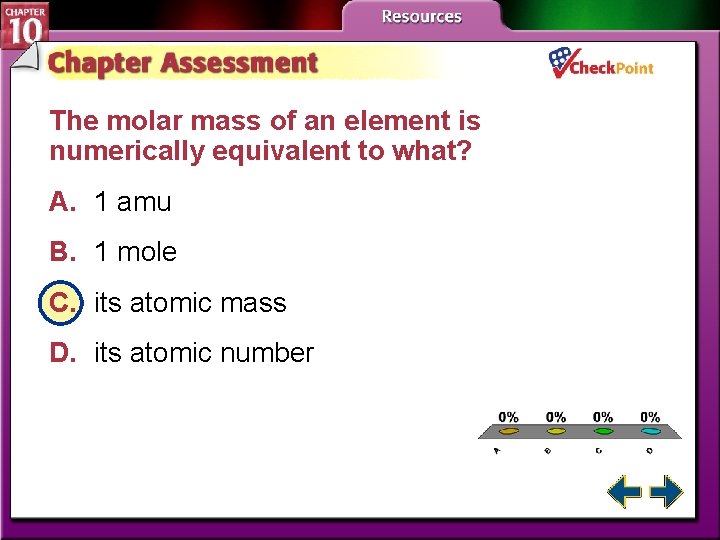
The molar mass of an element is numerically equivalent to what? A. 1 amu B. 1 mole C. its atomic mass D. its atomic number A. B. C. D. A B C D

How many moles of hydrogen atoms are in one mole of H 2 O 2? A. 1 B. 2 C. 3 D. 0. 5 A. B. C. D. A B C D

What is the empirical formula of Al 2 Br 3? A. Al. Br B. Al. Br 3 C. Al 2 Br D. Al 2 Br 3 A. B. C. D. A B C D

What is an ionic solid with trapped water molecules called? A. aqueous solution B. anhydrous compound C. hydrate D. solute A. B. C. D. A B C D

Two substances have the same percent by mass composition, but very different properties. They must have the same ____. A. density B. empirical formula C. molecular formula D. molar mass A. B. C. D. A B C D

How many moles of Al are in 2. 0 mol of Al 2 Br 3? A. 2 B. 4 C. 6 D. 1 A. B. C. D. A B C D

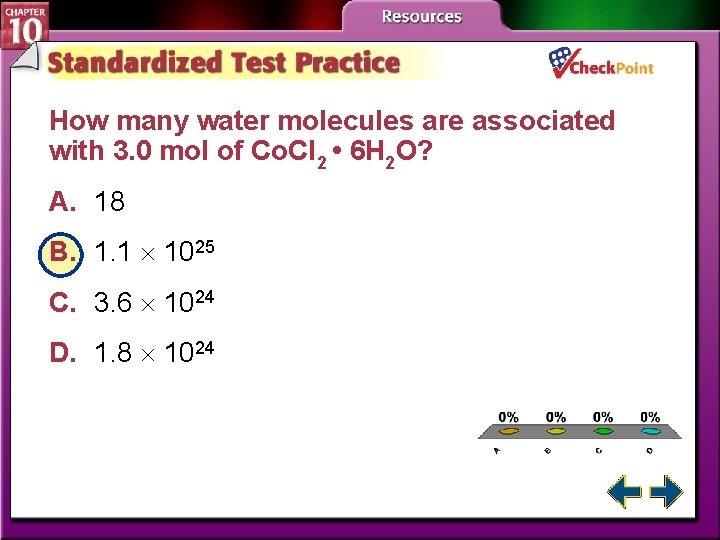
How many water molecules are associated with 3. 0 mol of Co. Cl 2 • 6 H 2 O? A. 18 B. 1. 1 1025 C. 3. 6 1024 D. 1. 8 1024 A. B. C. D. A B C D

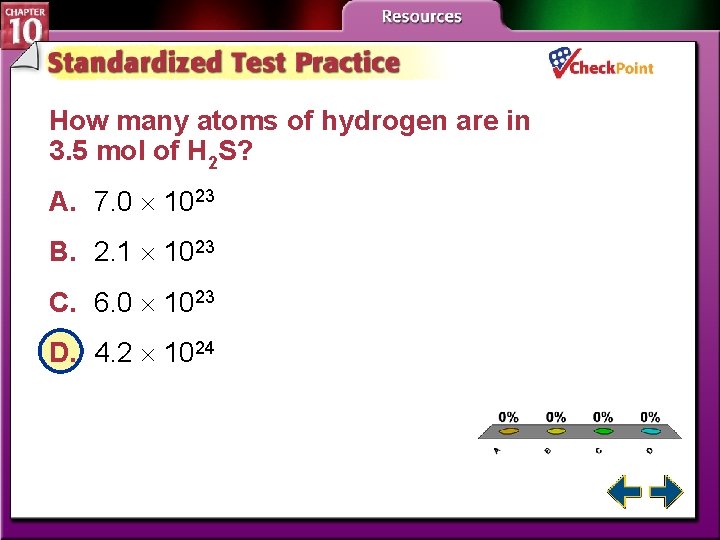
How many atoms of hydrogen are in 3. 5 mol of H 2 S? A. 7. 0 1023 B. 2. 1 1023 C. 6. 0 1023 D. 4. 2 1024 A. B. C. D. A B C D

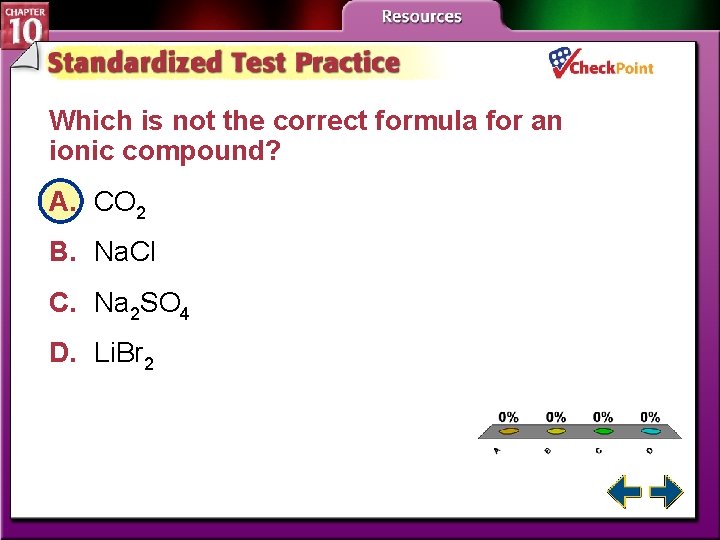
Which is not the correct formula for an ionic compound? A. CO 2 B. Na. Cl C. Na 2 SO 4 D. Li. Br 2 A. B. C. D. A B C D
