Section 1 Ion Formation Ions are formed when

- Slides: 10

Section 1: Ion Formation Ions are formed when atoms gain or lose valence electrons to achieve a stable octet electron configuration. K What I Know W What I Want to Find Out L What I Learned

Essential Questions • What holds atoms together in a chemical bond? • How do positive and negative ions form? • How does ion formation relate to electron configuration? Copyright © Mc. Graw-Hill Education Ion Formation

Vocabulary Review New • octet rule • chemical bond • cation • anion Copyright © Mc. Graw-Hill Education Ion Formation

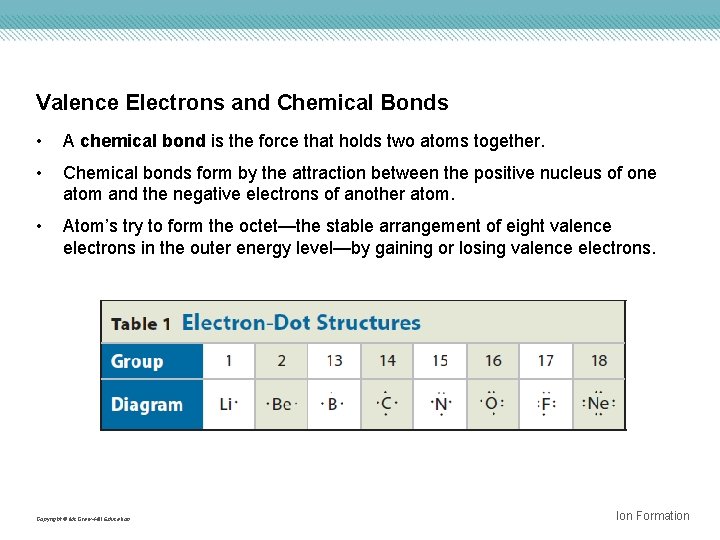
Valence Electrons and Chemical Bonds • A chemical bond is the force that holds two atoms together. • Chemical bonds form by the attraction between the positive nucleus of one atom and the negative electrons of another atom. • Atom’s try to form the octet—the stable arrangement of eight valence electrons in the outer energy level—by gaining or losing valence electrons. Copyright © Mc. Graw-Hill Education Ion Formation

Interactive Table – Electron-Dot Structures Concepts in Motion FPO Add link to concepts in motion animation from page 207 here. Copyright © Mc. Graw-Hill Education Ion Formation

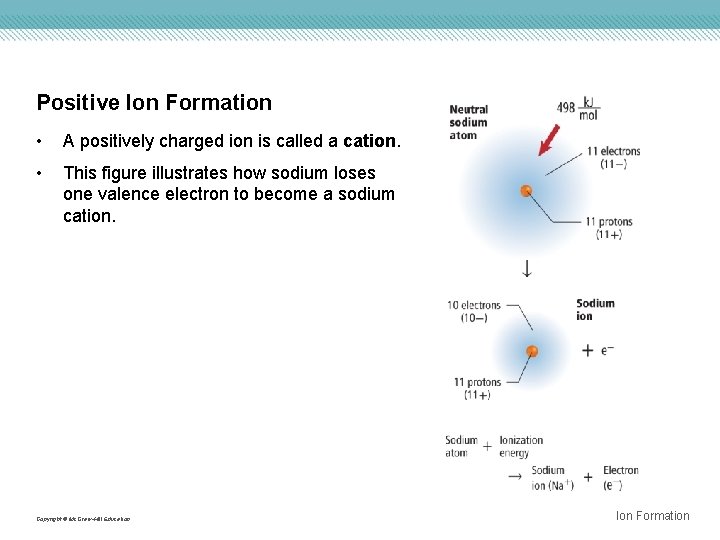
Positive Ion Formation • A positively charged ion is called a cation. • This figure illustrates how sodium loses one valence electron to become a sodium cation. Copyright © Mc. Graw-Hill Education Ion Formation

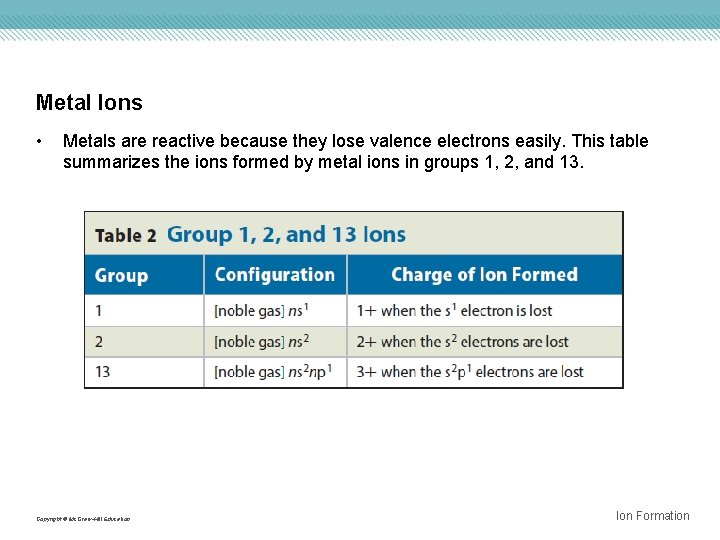
Metal Ions • Metals are reactive because they lose valence electrons easily. This table summarizes the ions formed by metal ions in groups 1, 2, and 13. Copyright © Mc. Graw-Hill Education Ion Formation

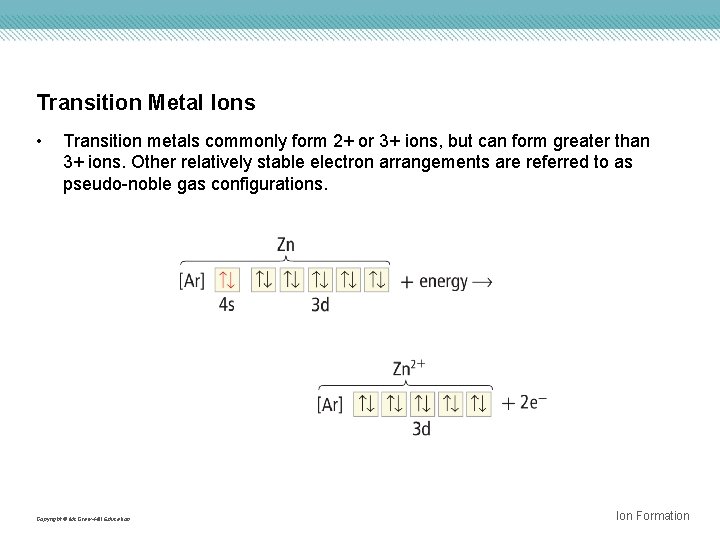
Transition Metal Ions • Transition metals commonly form 2+ or 3+ ions, but can form greater than 3+ ions. Other relatively stable electron arrangements are referred to as pseudo-noble gas configurations. Copyright © Mc. Graw-Hill Education Ion Formation

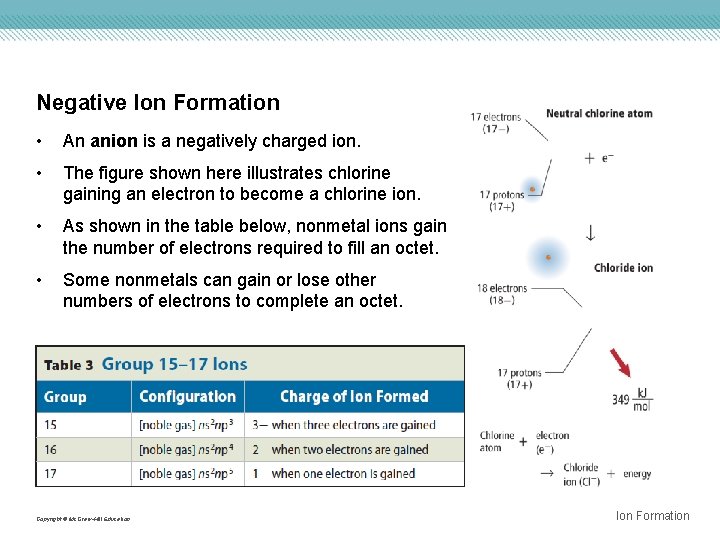
Negative Ion Formation • An anion is a negatively charged ion. • The figure shown here illustrates chlorine gaining an electron to become a chlorine ion. • As shown in the table below, nonmetal ions gain the number of electrons required to fill an octet. • Some nonmetals can gain or lose other numbers of electrons to complete an octet. Copyright © Mc. Graw-Hill Education Ion Formation

Review Essential Questions • What holds atoms together in a chemical bond? • How do positive and negative ions form? • How does ion formation relate to electron configuration? Vocabulary • chemical bond Copyright © Mc. Graw-Hill Education • anion Ion Formation