Section 1 6 WaveParticle Duality Electrons and all



- Slides: 10

Section 1. 6 – Wave-Particle Duality

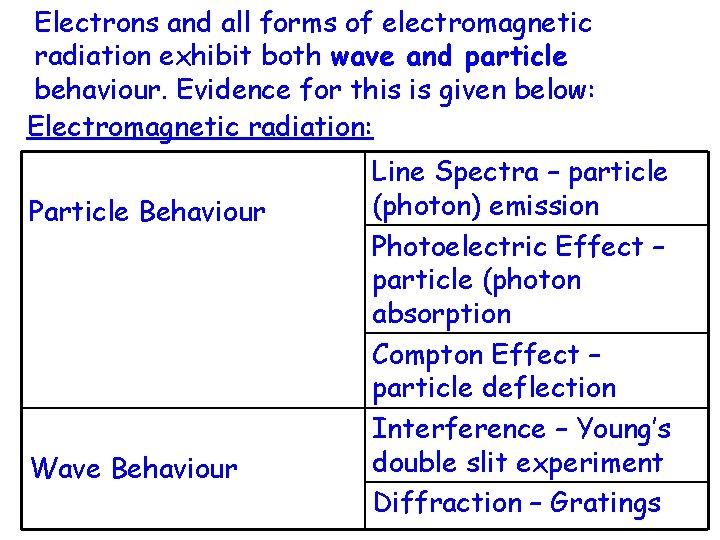
Electrons and all forms of electromagnetic radiation exhibit both wave and particle behaviour. Evidence for this is given below: Electromagnetic radiation: Particle Behaviour Wave Behaviour Line Spectra – particle (photon) emission Photoelectric Effect – particle (photon absorption Compton Effect – particle deflection Interference – Young’s double slit experiment Diffraction – Gratings

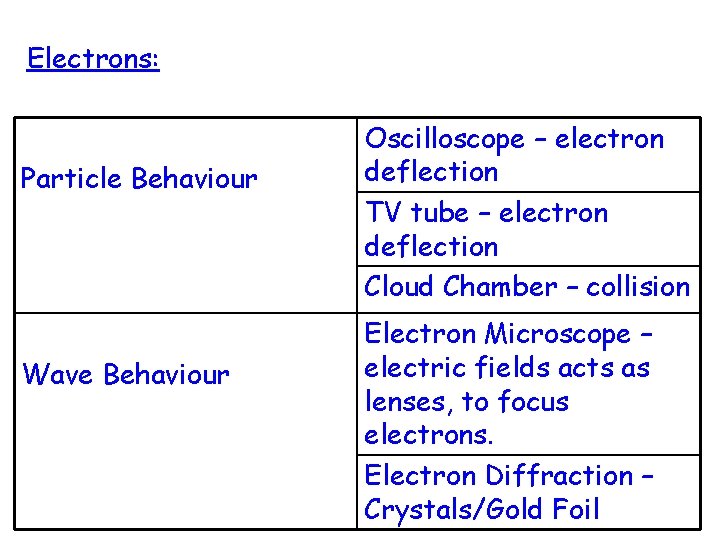
Electrons: Particle Behaviour Wave Behaviour Oscilloscope – electron deflection TV tube – electron deflection Cloud Chamber – collision Electron Microscope – electric fields acts as lenses, to focus electrons. Electron Diffraction – Crystals/Gold Foil

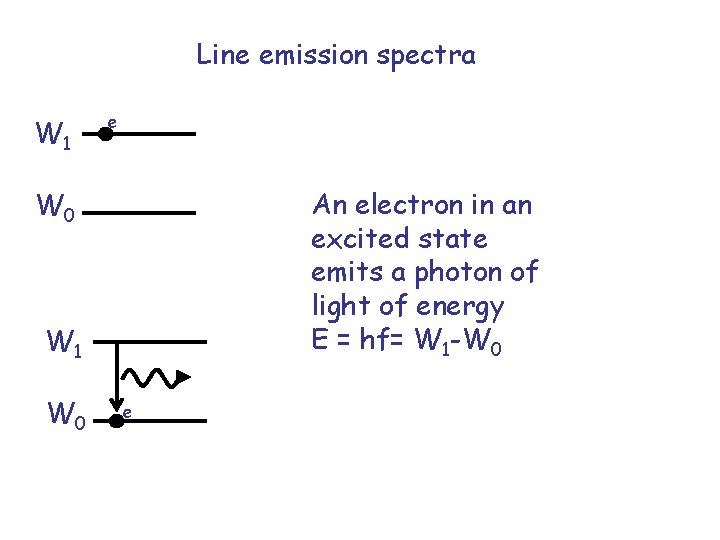
Line emission spectra W 1 e An electron in an excited state emits a photon of light of energy E = hf= W 1 -W 0 W 1 W 0 e

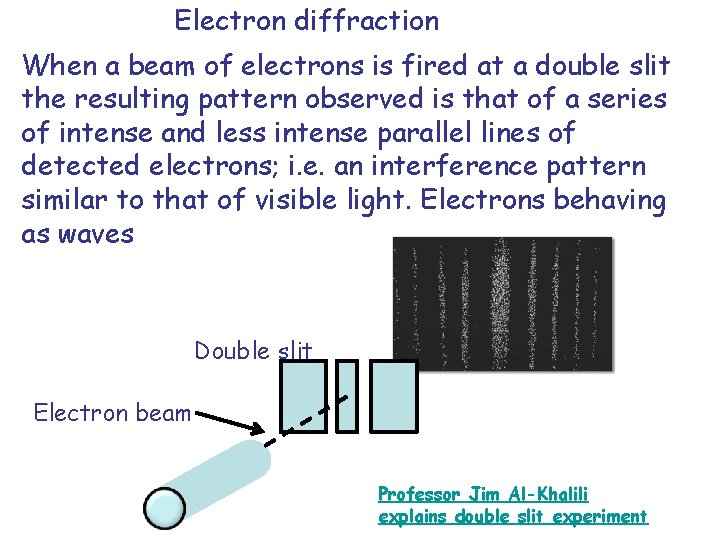
Electron diffraction When a beam of electrons is fired at a double slit the resulting pattern observed is that of a series of intense and less intense parallel lines of detected electrons; i. e. an interference pattern similar to that of visible light. Electrons behaving as waves Double slit Electron beam Professor Jim Al-Khalili explains double slit experiment

Both wave and particle behaviour cannot be observed at the same time so if we try to observe the electron passing through one of the slits (particle behaviour) then we immediately cause the interference pattern to collapse and only one or two dots appear directly in front of the slits.

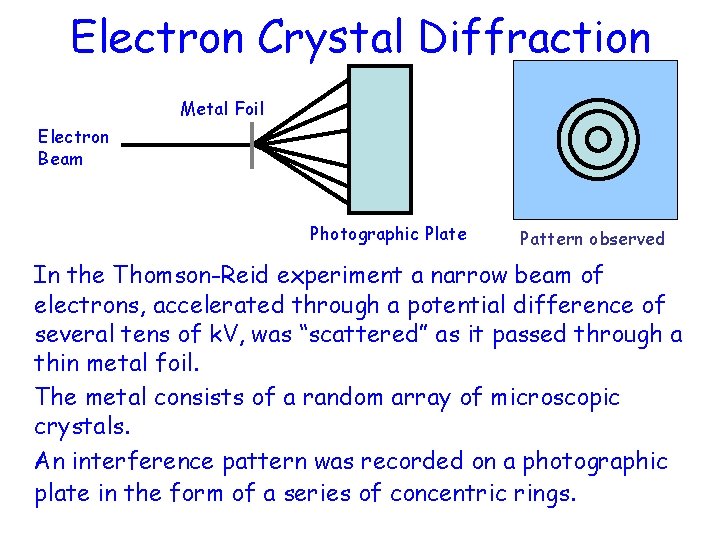
Electron Crystal Diffraction Metal Foil Electron Beam Photographic Plate Pattern observed In the Thomson-Reid experiment a narrow beam of electrons, accelerated through a potential difference of several tens of k. V, was “scattered” as it passed through a thin metal foil. The metal consists of a random array of microscopic crystals. An interference pattern was recorded on a photographic plate in the form of a series of concentric rings.

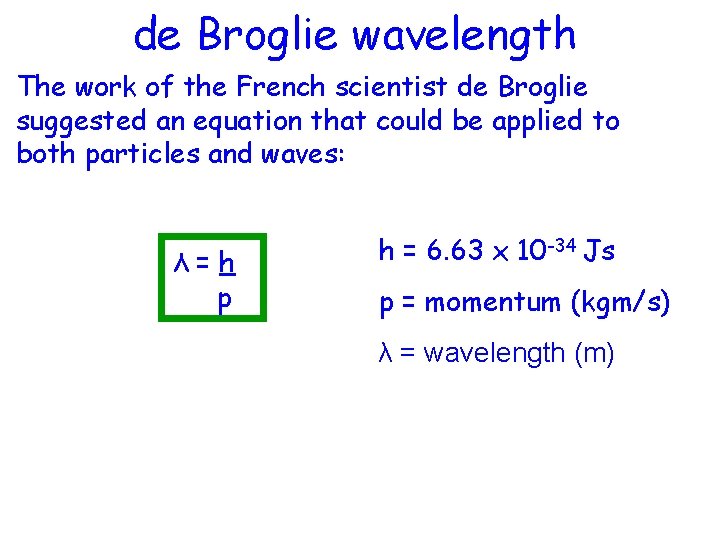
de Broglie wavelength The work of the French scientist de Broglie suggested an equation that could be applied to both particles and waves: λ=h p h = 6. 63 x 10 -34 Js p = momentum (kgm/s) λ = wavelength (m)

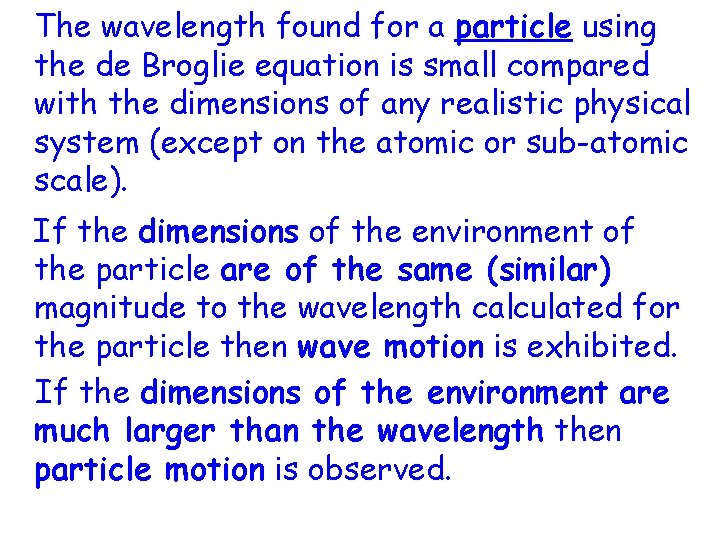
The wavelength found for a particle using the de Broglie equation is small compared with the dimensions of any realistic physical system (except on the atomic or sub-atomic scale). If the dimensions of the environment of the particle are of the same (similar) magnitude to the wavelength calculated for the particle then wave motion is exhibited. If the dimensions of the environment are much larger than the wavelength then particle motion is observed.

Example. a) A 50 kg boy runs at 4 m/s at a door 1 metre wide. Confirm if wave or particle nature is observed for the boy. b) An electron is accelerated through 2. 0 k. V and passes through a crystal of atomic separation 1. 0 x 10 -11 m. Confirm if wave or particle nature is observed.