Secondtrimester maternal serum screening Counseling for all patients




















- Slides: 22

Second-trimester maternal serum screening

Counseling for all patients Information about the screening tests offered – Detection rate – False-positive rate – Advantages – Disadvantages – Limitations Risks and benefits associated with diagnostic procedures

Goal for screening tests: §High detection rates §Low false-positive rates §Provide patients with the diagnostic options §they might to consider ØScreening andwant invasive diagnostic testing should be available to all women who present for prenatal care before 20 weeks of gestation regardless of maternal age.

ØFirst trimester screening with both NT and biochemical markers is an effective screening test for the general population, and is comparable to the 2 nd trimester quadruple screen with the additional advantage of earlier pregnancy terminations if desired. ØNeural tube defect screening should be offered in the second trimester to women who elect only first trimester screening for aneuploidy

Second Trimester Quad Screen § 15 -22 weeks gestation §AFP, h. CG, u. E 3, inhibin A § 81% DR at a 5% positive screen rate §Good for women who don’t want 1 st trimester screening or who register

Used for detection of: n of : 1. ONTD 2. Down syndrome 3. trisomy 18 4. Smith-Lemli-Opitz syndrome

ØHyperglycosylated h. CG excreted in maternal urine has been tested as a marker for Down syndrome ØWith the addition of extra markers, the potential benefit must be balanced against the cost.

Screening tests that combine first and second-trimester markers ØIntegrated ØSequential – Stepwise sequential – Contingent sequential

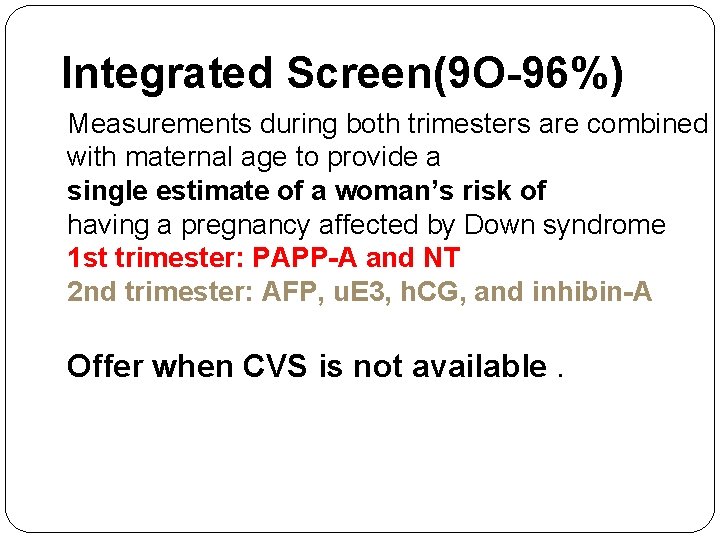
Integrated Screen(9 O-96%) Measurements during both trimesters are combined with maternal age to provide a single estimate of a woman’s risk of having a pregnancy affected by Down syndrome 1 st trimester: PAPP-A and NT 2 nd trimester: AFP, u. E 3, h. CG, and inhibin-A Offer when CVS is not available.

Sequential Screening Testsis informed of the first-trimester screening ØPatient result ØAllows patients the option to have CVS – Stepwise Sequential Screening – Contingent Sequential Screeni ng Ø 88 -94% DR at a 5% positive screen rate

Stepwise Sequential Screen 1 st trimester: NT, PAPP-A + maternal age(90%-95%) Risk Assessment Positive 1% Negative(99%) CVS Quad screen Risk based on maternal age, 1 st trimester + quad screen

Contingent Sequential Screen(8894%) 1 st trimester: NT, PAPP-A + maternal age Øscreen positive → offer CVS(1%) Øintermediate → offer quad screen(15%) Øscreen negative → no further testing(84%)

ABNORMAL SECOND-TRIMESTER MATERNAL SERUM MARKERS IN PREGNANCIES WITH A NORMAL KARYOTYPE Unexplained Elevated Maternal Serum αFetoprotein(2. 5 MOM) §Ultrasound ONTD screening § 90% r/o spinal lesions § 100% r/o anencephaly §VWD reduced with normal scan §If MSAFP >4. 0 Mo. M and NL U/S Offe invasive testing §fetal growth restriction, §fetal death, § prematurity, §oligohydramnios, §abruptio placentae, and preeclampsia. Øno management protocol has been demonstrated to improve outcome in these cases.

Several factors influence the maternal serum AFP level and are taken into consideration when calculating the AFP Mo. M: ØMaternal weight ØGestational age ØRace or ethnicity ØDiabetes ØMultifetal gestation

Unexplained Elevated Human Chorionic Gonadotropin Levels unexplained elevated h. CG (>2. 0 Mo. M) is associated with an increased risk for preeclampsia, preterm birth, low birth weight, fetal demise, and possibly hypertension Low Second-Trimester Maternal Serum Estriol. Low maternal serum unconjugated estriol levels have been linked to adverse pregnancy outcomes Very low or absent estriol levels of 0. 0 to 0. 15 Mo. M suggest biochemical abnormalities of the fetus or placenta, including placental steroid sulfatase deficiency, Smith-Lemli-Opitz syndrome, congenital adrenal hypoplasia, adrenocorticotropin deficiency, hypothalamiccorticotropin deficiency, and

Smith-Lemli-Opitz syndrome occurs in approximately 1/60, 000 pregnancies and is an autosomal recessive disorder defect in 3β-hydroxysteroid-Δ 7 -reductase, altering cholesterol synthesis and resulting in low cholesterol levels and the accumulation of the cholesterol precursor 7 dehydrocholesterol in blood and amniotic fluid. Smith-Lemli-Opitz syndrome is characterized by low birth weight, failure to thrive, and moderate to severe mental retardation. It is associated with multiple including syndactyly structural anomalies, of the second and third toes, microcephaly, ptosis, and a typical-appearing facies. Undermasculinization of the genitalia, including complete sex reversal, can be

Elevated Human Chorionic Gonadotropin and Maternal Serum α-Fetoprotein The combination of elevated MSAFP and h. CG levels occurs rarely but may have an overall pregnancy complication rate exceeding 50%. preeclampsia, preterm birth, growth restriction, placental abnormalities, and fetal death Confined placental mosaicism for chromosome 16 has been reported to be associated with extremely high levels of both analytes, as well as with similarly poor outcomes.

Abnormal Quad Screen Markers ↑ed inhibin A + ↑ed h. CG and/or AFP (>2. 0 Mo. M): Consider: ØUterine artery Dopplers at @18 -20 weeks ØClose surveillance for IUGR and preeclampsia ØNon-stress tests for cases with IUGR or preeclampsia

Down Syndrome Screening for Monozygotic Twins ØRisk for aneuploidy is the same as for singleton ØAverage of the two NT measurements can be used to calculate the pregnancy risk ØAn enlarged NT and/or significantly discordant measurements between twins may be markers for adverse outcomes independent of aneuploidy risk

Down Syndrome Screening for Dizygotic Twins (> 70% of twins) ØEach fetus carries an independent risk for Down syndrome ØAge-related risk for having 1 aneuploid fetus is higher than that of a woman with a singleton pregnancy ØIndividual NT measurements can be used to calculate the fetus-specific risk ØA maternal age of 31 -33 years is the equivalent risk of a 35 -year old woman with a singleton

Screening Tests in Twins For twins, however, the value and accuracy of serum screening is much less certain because the contribution of an abnormal fetus will, on average, be brought closer to the normal mean by an unaffected co-twin. This tends to decrease the overall screening sensitivity. Screening, however, can be useful nonetheless. At present, there is no standard agreement on the MSAFP elevation that warrants further evaluation in twins. Some centers use a cutoff of 4. 0 Mo. M, which would identify approximately 60% of fetuses with open spina bifida, but this has approximately an 8% incidence of false-positive results. Other centers use a cutoff of 4. 5 50%. Mo. M, which has a sensitivity of approximately
