Second Line ARV Doses Side Effects HAIVN Harvard














- Slides: 29

Second Line ARV: Doses & Side Effects HAIVN Harvard Medical School AIDS Initiative in Vietnam

Learning Objectives At the end of this lecture, each trainee should know: • The second-line ARV regimen recommended by the Vietnam MOH • The common side effects of second line drugs • How to manage side effects 2

Content • Second-line ARV regimen • Second-line ARV drugs § Formulations § Dosing § Common Side effects 3

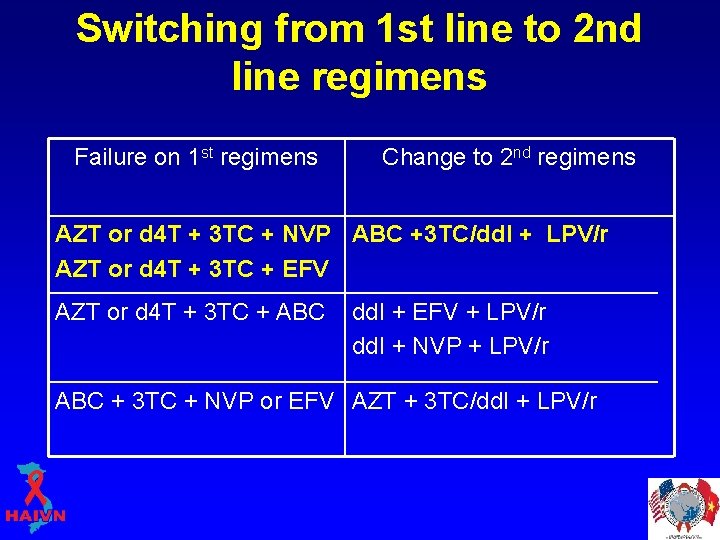
Switching from 1 st line to 2 nd line regimens Failure on 1 st regimens Change to 2 nd regimens AZT or d 4 T + 3 TC + NVP ABC +3 TC/dd. I + LPV/r AZT or d 4 T + 3 TC + EFV AZT or d 4 T + 3 TC + ABC dd. I + EFV + LPV/r dd. I + NVP + LPV/r ABC + 3 TC + NVP or EFV AZT + 3 TC/dd. I + LPV/r

Second-Line ARV • NRTIs § Abacavir (ABC) § Didanosine (dd. I) • Protease Inhibitors § Lopinavir / ritonavir (LPV/r)

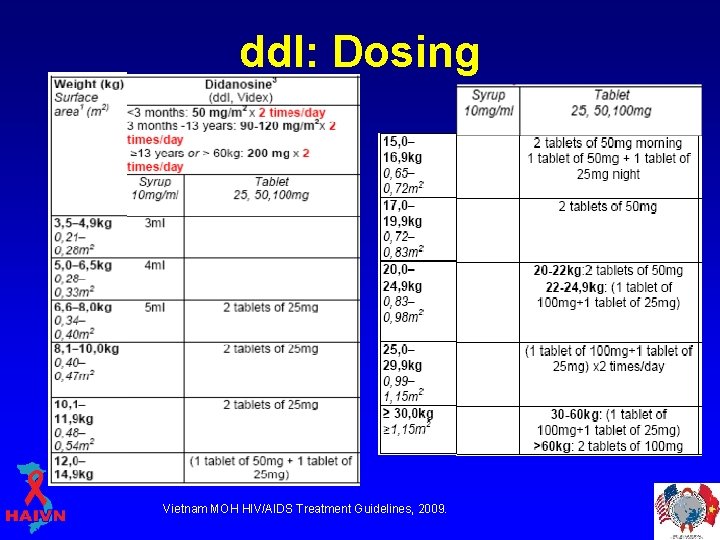
Didanosine (dd. I; Videx) • Formulations*: § Liquid mixed in antacid (usually Maalox) § Chewable/dispersible tablet (with an antacid buffer) § Extended release, enteric coated capsule (Videx EC) * Absorption of dd. I in the stomach is affected by acid.

dd. I: Dosing Vietnam MOH HIV/AIDS Treatment Guidelines, 2009.

dd. I: Pharmacokinetics • DDI should be given on an empty stomach (1 hour before or 2 hours after a meal) • DDI liquid should be stored under refrigerated conditions • DDI tablets: minimum dose is two tablets at a time. § Tablets contain an antacid and two tablets are needed to supply enough antacid to provide adequate buffering. • EC capsules: enteric coating protects the dd. I during its transport through the stomach

dd. I: Side Effects • Peripheral neuropathy: § Presents as paresthesias, most often of the feet § May appear as a gait disturbance in younger children • Pancreatitis: § Presents as abdominal pain, nausea, and vomiting § Associated with a rise in amylase and lipase levels • Lipoatrophy: § d 4 T+dd. I > d 4 T > dd. I • Lactic acidosis: § d 4 T+dd. I > d 4 T > dd. I

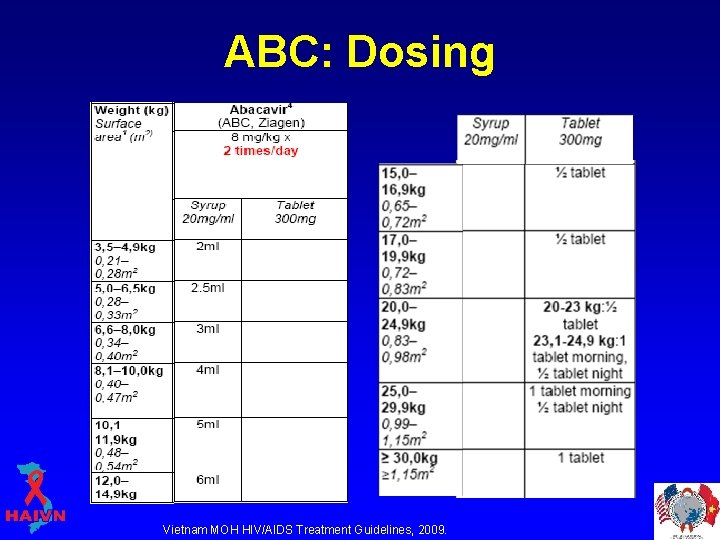
Abacavir (ABC; Ziagen) • Formulations: § Capsule 300 mg § Syrup 20 mg/ml • Administered on an every 12 -hour schedule. • There is no food effect on absorption.

ABC: Dosing Vietnam MOH HIV/AIDS Treatment Guidelines, 2009.

Abacavir – Hypersensitivity • Incidence: 3 - 6% • Time of presentation: § median = 11 th day § 93% of cases occur in the first 6 weeks • Clinical symptoms: § Most common: fever, maculopapule rash, fatigue § GI Symptoms: nausea, vomiting, diarrhea, abdominal pain § Respiratory symptoms: cough, shortness of breath • Contraindications: § Previous ABC hypersensitivity • Hypotension or death upon re-challenge! Patients with hypersensitivity should never receive ABC again! 12

Abacavir Hypersensitivity – Laboratory Abnormalities • Common § Lymphopenia: redistribution effect § elevated liver enzymes • Less frequent § § elevated creatine phosphokinase: may be pronounced mild thrombocytopenia: not clinically significant renal: increased serum creatinine lungs: chest X-ray may be normal or display diffuse bilateral or lobar infiltrates • Laboratory abnormalities resolve a few days after discontinuing abacavir 13

Abacavir Hypersensitivity – Treatment • Stop Abacavir immediately if hypersensitivity is suspected § Symptoms will usually improve within a few days § Never give Abacavir again § Note Abacavir hypersensitivity in the patient record § Notify the patient and caregiver of the reaction and counsel them not to take abacavir again • For severe reactions or hypotension: § Admit to hospital or ICU 14

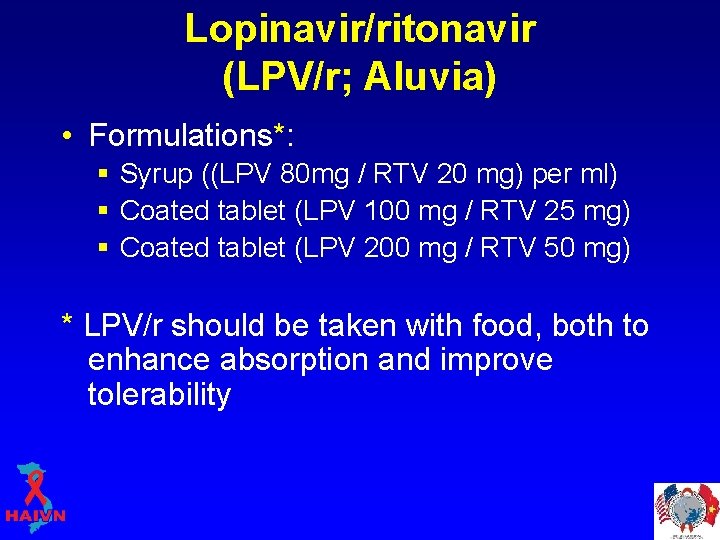
Lopinavir/ritonavir (LPV/r; Aluvia) • Formulations*: § Syrup ((LPV 80 mg / RTV 20 mg) per ml) § Coated tablet (LPV 100 mg / RTV 25 mg) § Coated tablet (LPV 200 mg / RTV 50 mg) * LPV/r should be taken with food, both to enhance absorption and improve tolerability

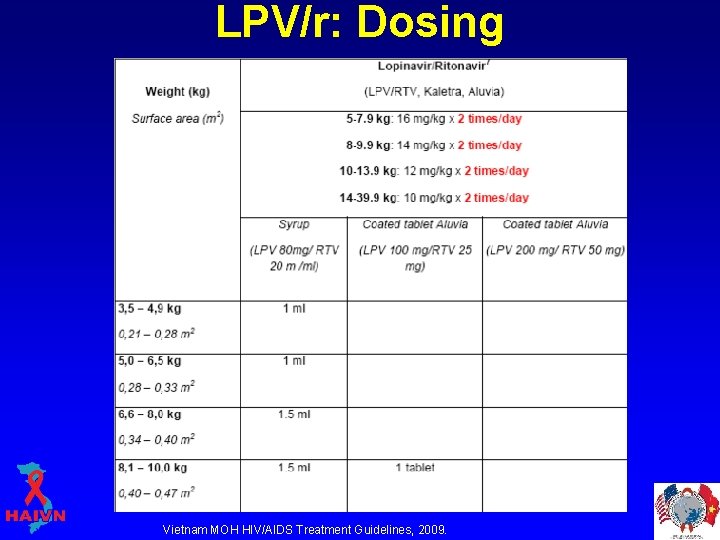
LPV/r: Dosing Vietnam MOH HIV/AIDS Treatment Guidelines, 2009.

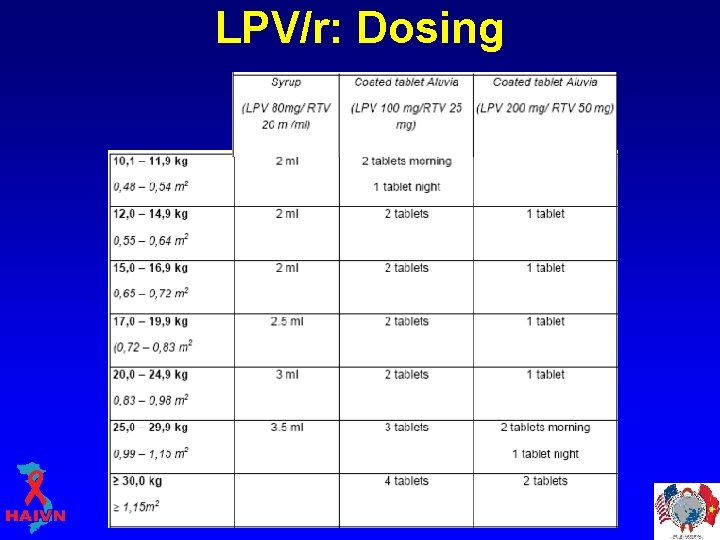
LPV/r: Dosing

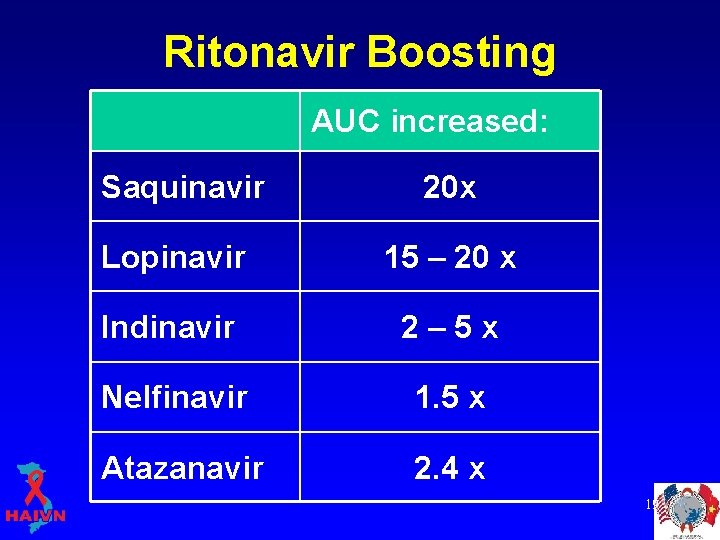
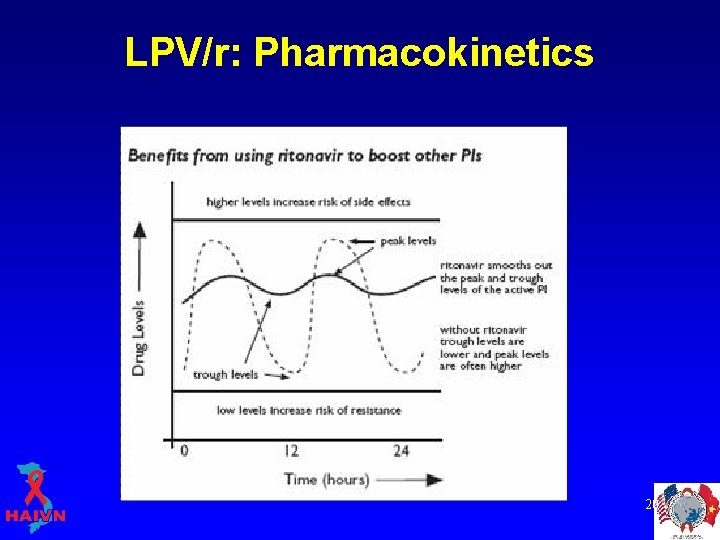
LPV/r: Pharmacokinetics Ritonavir boosting • Most potent liver enzyme (Cytochrome P 450 3 A 4) inhibitor available § inhibits the break down of drugs in the liver, including other protease inhibitors • Now used to boost other PIs § § “boosted PI” Increases AUC of the other PI Can use lower dose of other PI Increases the Cmin (trough concentration) of other PI, decreasing the risk for resistance § Allows once or twice daily dosing of PI 18

Ritonavir Boosting AUC increased: Saquinavir 20 x Lopinavir 15 – 20 x Indinavir 2– 5 x Nelfinavir 1. 5 x Atazanavir 2. 4 x 19

LPV/r: Pharmacokinetics 20

LPV/r: Drug interactions • Rifampin: LPV by 75% § Generally should avoid combination § If necessary can give additional ritonavir to “super” boost the LPV (consult with expert) • Clarithromycin levels § Adjust clarithromycin dose only in renal failure • Itraconazole levels § Use with caution at itraconazole doses > 200 mg/day

LPV/r: Side Effects • GI intolerance (nausea, vomiting) • Diarrhea • Long-term side effects: § Fat accumulation (lipodystrophy) § Lipids: increased cholesterol, triglycerides § Insulin resistance, increased glucose

LPV/r Side Effects: Lidodystrophy • Changes in body fat distribution (lipodystrophy) have been reported to occur in 1%– 33% of children with HIV infection. • Lipohypertrophy or central fat accumulation is most often associated with protease inhibitor therapy. • In children, physical examination showa increased abdominal girth, dorso-cervical fat accumulation, and/or breast enlargement.

LPV/r Side Effects: Lidodystrophy • Development of lipohypertrophy is probably related to: § Genetic and developmental characteristics § Lifestyle factors (diet and exercise/activity) § ARV exposure and duration • Treatment: § Low-fat diet § Exercise § Switch PI to NNRTI (if not resistant to NNRTI already) • Support of the patient and family is an important aspect of care.

PI: Metabolic Effects • Elevated Cholesterol § Check lipids yearly (Cholesterol, LDL, HDL, Triglycerides) § Treatment: • • Dietary changes Exercise Lipid lowering drugs Change in ARV regimen if possible

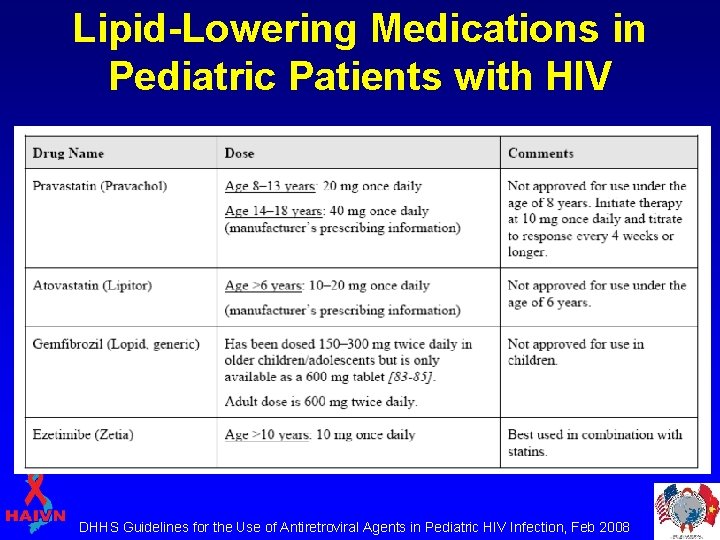
Lipid-Lowering Medications in Pediatric Patients with HIV DHHS Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection, Feb 2008

PI: Metabolic Effects • Insulin resistance, hyperglycemia § When starting PIs, caregivers and patients should be educated about the signs and symptoms of diabetes mellitus § Check glucose every 6 -12 months § Lifestyle modification if impaired glucose tolerance 27

Key Points • The MOH second-line ARV regimens for children is ABCDDI-LPV/r • Patients with abacavir hypersensitivity should never take abacavir again due to the risk of recurrent reaction and death. • Be aware of potential drug interactions with patients on LPV/r. • Patients on LPV/r should have glucose and lipid tests done at least once a year to screen for metabolic side effects. 28

Thank you! Questions?