Second Law of Thermodynamics Introduction Random Kinetic Energy











- Slides: 25

Second Law of Thermodynamics • • Introduction Random Kinetic Energy and Probability Early Observations Kelvin-Planck and Clausius statements Entropy statement Allowed/Disallowed processes Examples

Second Law of Thermodynamics • What “drives” heat? – Heat flows Q = (k. A/l) ΔT – But always flows from hot to cold. • What’s the nature of this “driver”? – – – Force? - gravitation? Force? - electrostatic? No! - a desire to “spread out”, distribute more evenly Random kinetic energy – governed by statistics Go to most probable state. • Most probable is intermixed - requires flow from “Hot” to “Cold”. http: //en. wikipedia. org/wiki/Statistical_mechanics

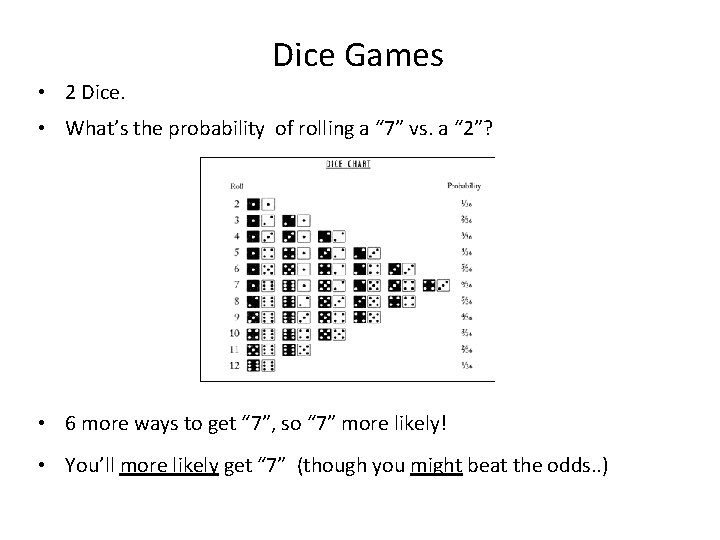
Dice Games • 2 Dice. • What’s the probability of rolling a “ 7” vs. a “ 2”? • 6 more ways to get “ 7”, so “ 7” more likely! • You’ll more likely get “ 7” (though you might beat the odds. . )

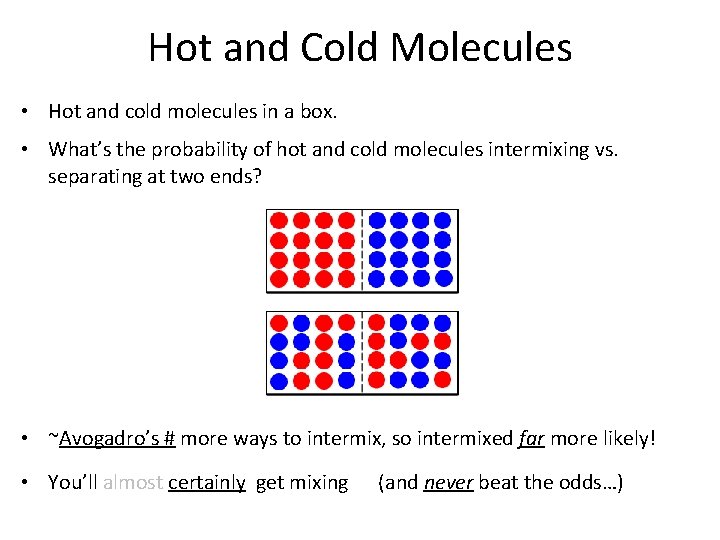
Hot and Cold Molecules • Hot and cold molecules in a box. • What’s the probability of hot and cold molecules intermixing vs. separating at two ends? • ~Avogadro’s # more ways to intermix, so intermixed far more likely! • You’ll almost certainly get mixing (and never beat the odds…)

2 nd Law - Early Observations 1. Cannot take heat and turn completely into work – Flow from hot to cold may be “diverted” to do work. – No perpetual motion machines. 2. Heat always flows from “Hot” to “Cold” – Hot gets colder, cold gets hotter – Never the other way!

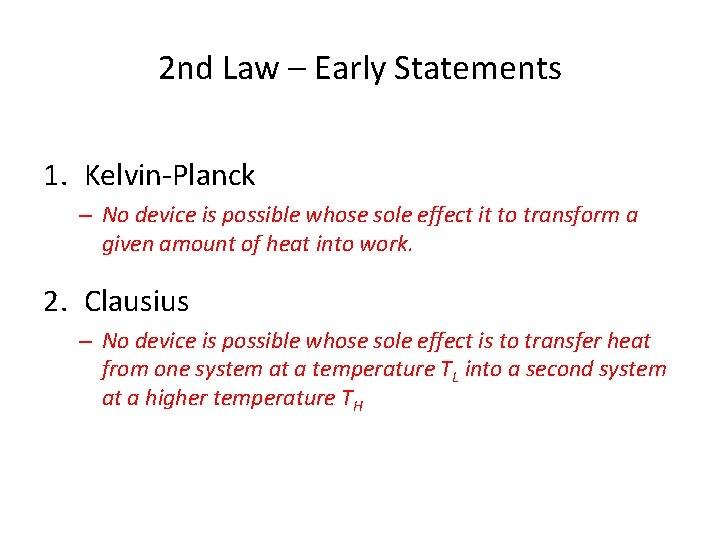
2 nd Law – Early Statements 1. Kelvin-Planck – No device is possible whose sole effect it to transform a given amount of heat into work. 2. Clausius – No device is possible whose sole effect is to transfer heat from one system at a temperature TL into a second system at a higher temperature TH

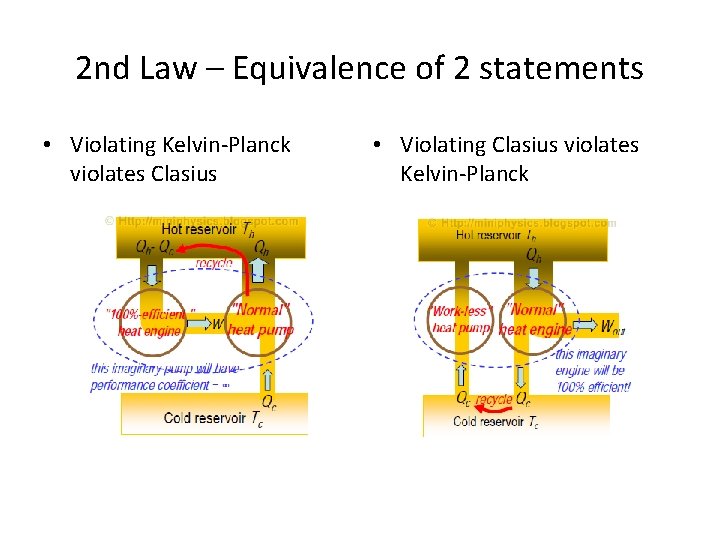
2 nd Law – Equivalence of 2 statements • Violating Kelvin-Planck violates Clasius • Violating Clasius violates Kelvin-Planck

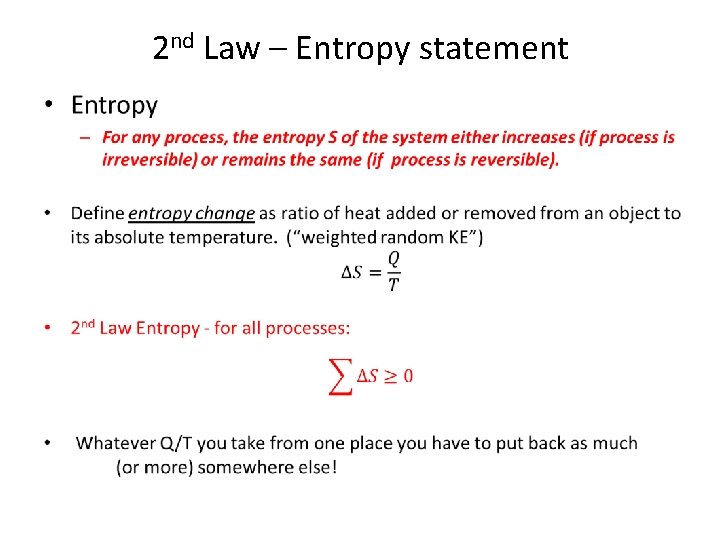
2 nd Law – Entropy statement •

2 nd Law Entropy - Examples 1. Can an ideal Carnot engine work? 2. Can a heat pump deliver more heat than it takes in? 3. Can heat flow spontaneously hot to cold? 4. Can heat flow spontaneously cold to hot? (Clausius) 5. Can heat be completely converted to work? (Kelvin-Planck) 6. Can the “Everything” problem work?

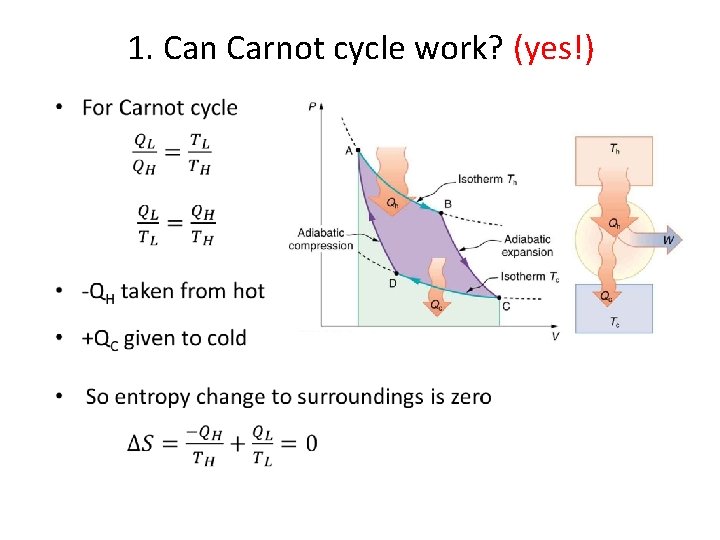
1. Can Carnot cycle work? (yes!) •

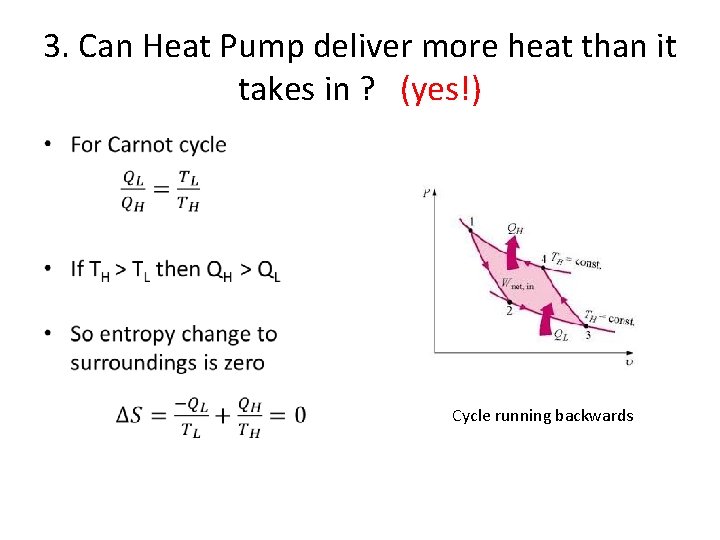
3. Can Heat Pump deliver more heat than it takes in ? (yes!) • Cycle running backwards

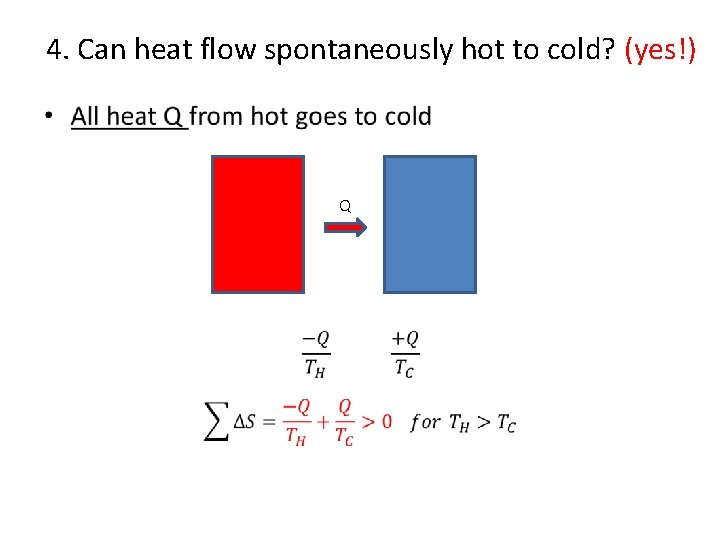
4. Can heat flow spontaneously hot to cold? (yes!) • Q

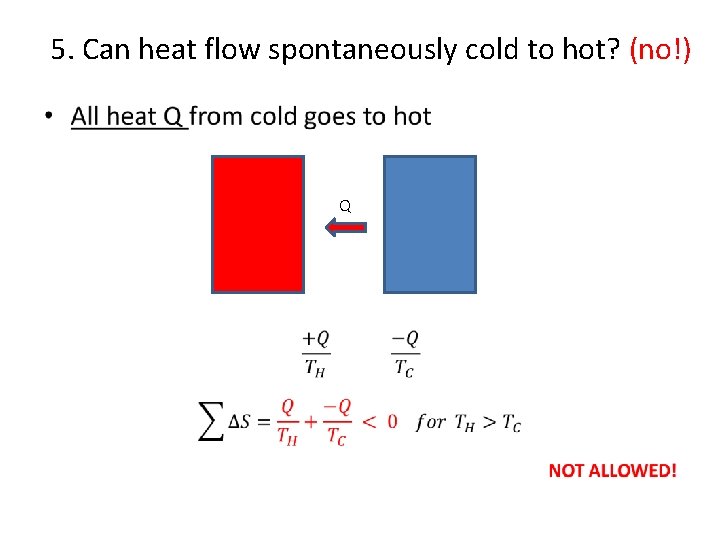
5. Can heat flow spontaneously cold to hot? (no!) • Q

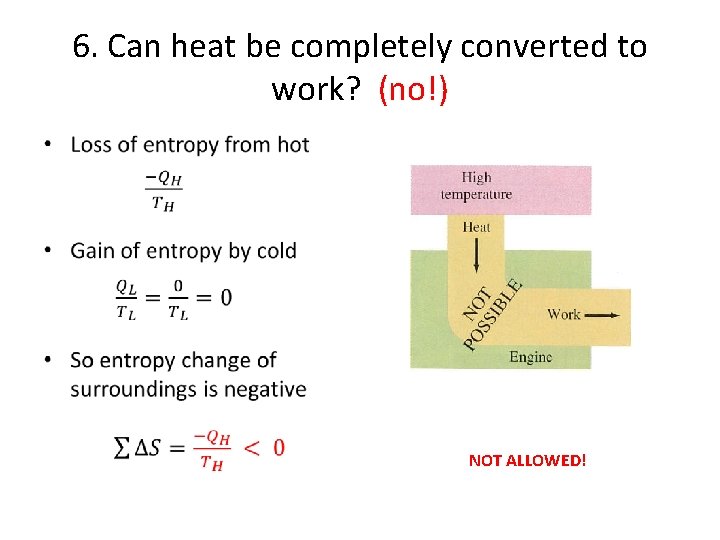
6. Can heat be completely converted to work? (no!) • NOT ALLOWED!

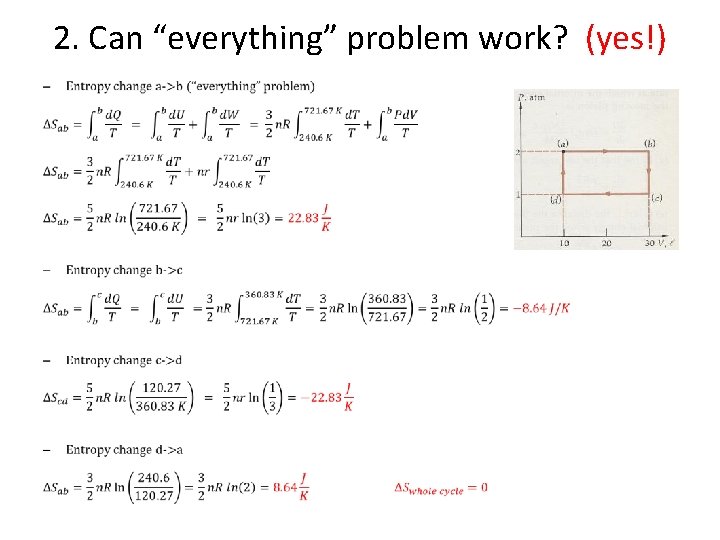
2. Can “everything” problem work? (yes!) •

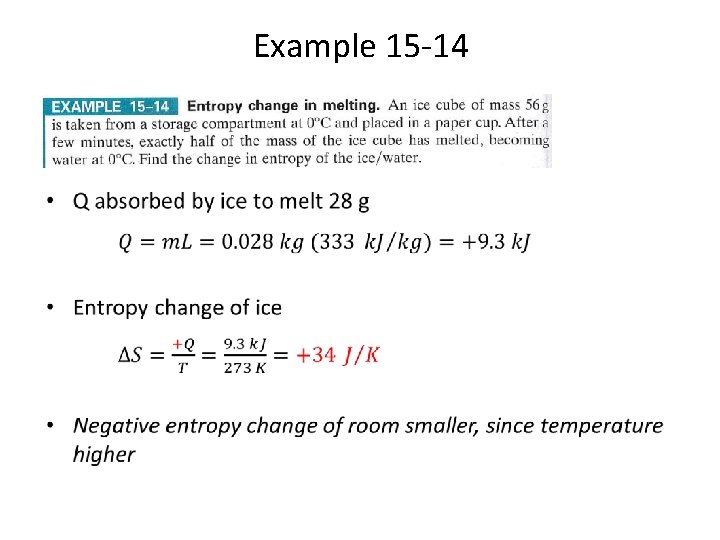
Example 15 -14 •

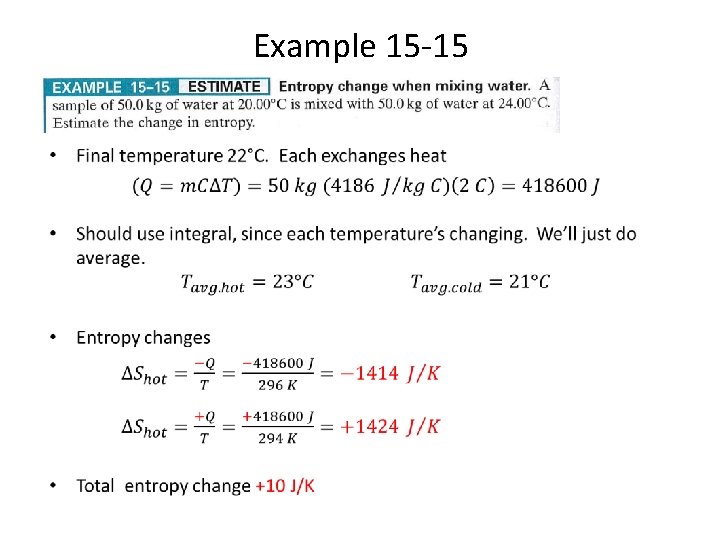
Example 15 -15 •

Problem 38 •

Problem 38 (cont) •

End Thermodynamics Next Electromagnetism

Spring Break Assignment 1. Go skiing 2. Go snowboarding 3. Take an early spring hike in a hilly area

Spring Break Assignment Notice 2 things: – Slope – Elevation And the 2 are related!

Slope • Slope in the east/west direction • Slope in the north/south direction • Maximum slope (fall line) • No slope (perpendicular) Like a vector!

Elevation • 7000, 7100 feet, etc • Just a single number (like a scalar) • When elevation changes rapidly slope is steep • When elevation changes slowly slope is gradual 7100 7000

Analogy with Chapters 16, 17 • Slope analogous to Electric Field! • Elevation analogous to Electric Potential!