Second Law of Thermodynamics 1 st and 2

- Slides: 9

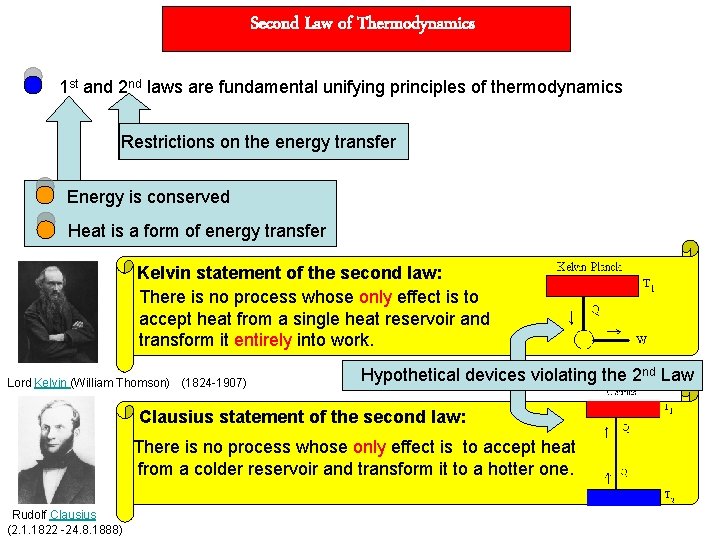
Second Law of Thermodynamics 1 st and 2 nd laws are fundamental unifying principles of thermodynamics Restrictions on the energy transfer Energy is conserved Heat is a form of energy transfer Kelvin statement of the second law: There is no process whose only effect is to accept heat from a single heat reservoir and transform it entirely into work. Lord Kelvin (William Thomson) (1824 -1907) Hypothetical devices violating the 2 nd Law Clausius statement of the second law: There is no process whose only effect is to accept heat from a colder reservoir and transform it to a hotter one. Rudolf Clausius (2. 1. 1822 -24. 8. 1888)

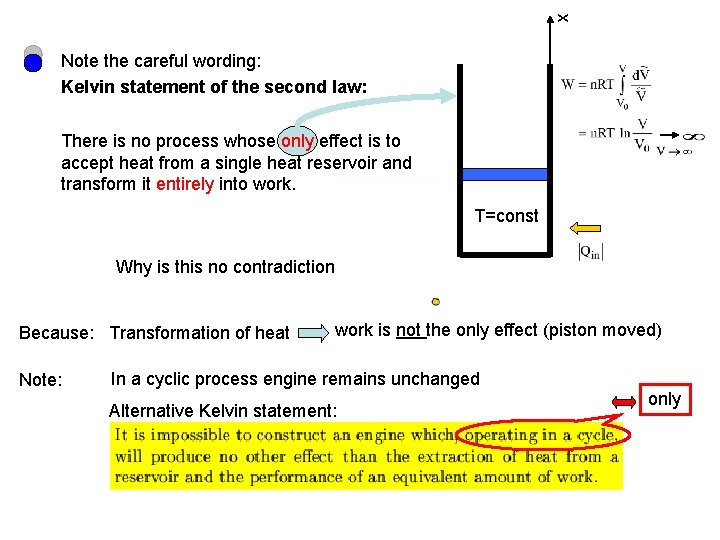
x Note the careful wording: Kelvin statement of the second law: There is no process whose only effect is to accept heat from a single heat reservoir and transform it entirely into work. T=const Why is this no contradiction Because: Transformation of heat Note: work is not the only effect (piston moved) In a cyclic process engine remains unchanged Alternative Kelvin statement: only

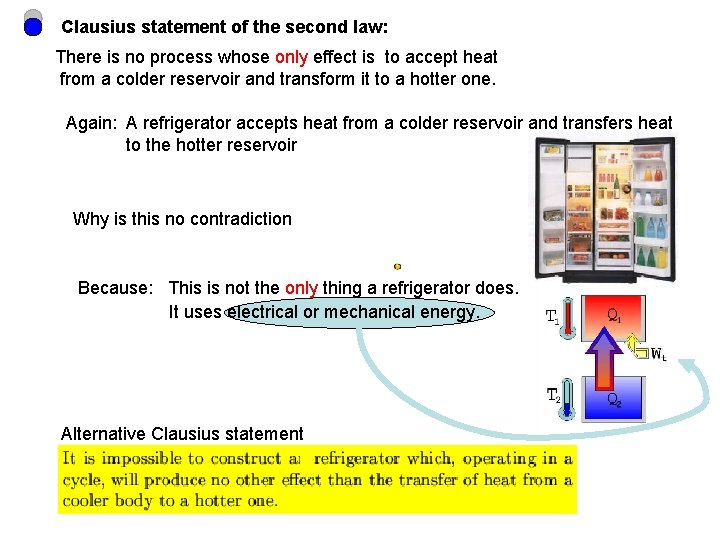
Clausius statement of the second law: There is no process whose only effect is to accept heat from a colder reservoir and transform it to a hotter one. Again: A refrigerator accepts heat from a colder reservoir and transfers heat to the hotter reservoir Why is this no contradiction Because: This is not the only thing a refrigerator does. It uses electrical or mechanical energy. Alternative Clausius statement

Michael Flanders and Donald Swan The First Law of Thermodynamics: Heat is work and work is heat. Very good! The Second Law of Thermodynamics: Heat cannot of itself pass from one body to a hotter body. Heat won’t pass from a cooler to a hotter. You can try it if you like, but you far better notter, Cause the cold in the cooler will get hotter as a ruler, Cause the hotter body’s heat will pass to the cooler. First law: Heat is work and work is heat. Heat will pass by conduction, And heat will pass by convection, And heat will pass by radiation, And that’s a physical law. Heat is work and work’s a curse, And all the heat in the universe is gonna to cool down, Cause it can’t increase, And then there’ll be no more work and there’ll be perfect peace. Really! Yeah, that’s entropy man. And all because of the second law of thermodynamics which lays down that: You can’t pass heat from a cooler to a hotter. You can try it if you like, but you’d far better notter, Cause the cold in the cooler will get hotter as a ruler, Cause the hotter body’s heat will pass to the cooler. And that’s a physical law. Ooh, I’m hot! Hot! That’s because you’ve been working! That’s the first and second law of thermodynamics.

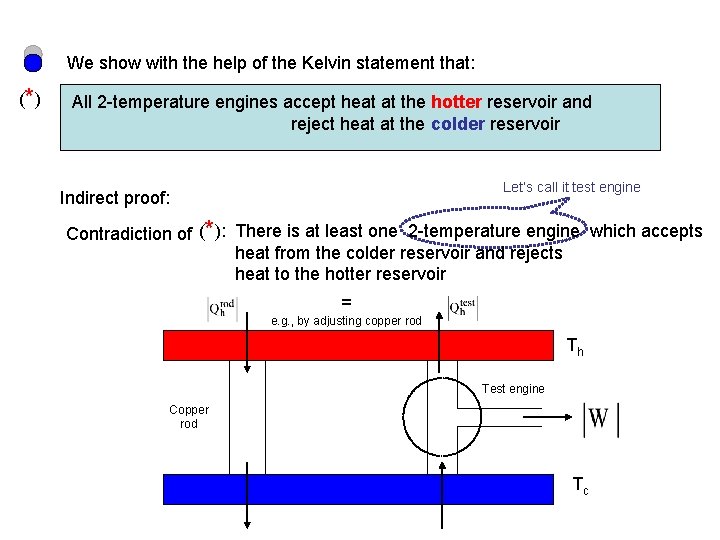
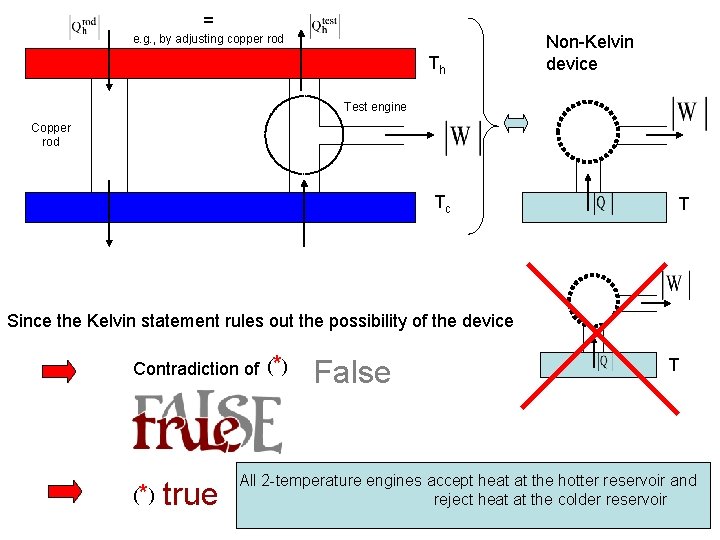
We show with the help of the Kelvin statement that: (* ) All 2 -temperature engines accept heat at the hotter reservoir and reject heat at the colder reservoir Let’s call it test engine Indirect proof: Contradiction of (*): There is at least one 2 -temperature engine which accepts heat from the colder reservoir and rejects heat to the hotter reservoir = e. g. , by adjusting copper rod Th Test engine Copper rod Tc

= e. g. , by adjusting copper rod Th Non-Kelvin device Test engine Copper rod Tc T Since the Kelvin statement rules out the possibility of the device Contradiction of (*) (* ) true False T All 2 -temperature engines accept heat at the hotter reservoir and reject heat at the colder reservoir

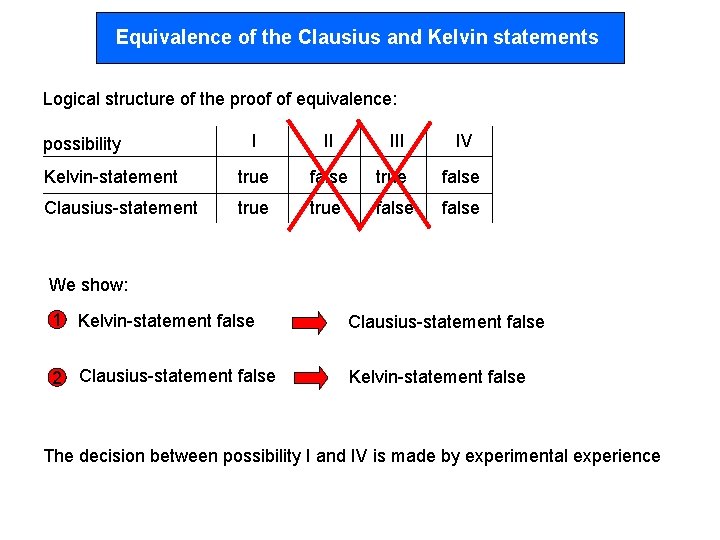
Equivalence of the Clausius and Kelvin statements Logical structure of the proof of equivalence: I II Kelvin-statement true false Clausius-statement true false possibility III IV We show: 1 Kelvin-statement false Clausius-statement false 2 Clausius-statement false Kelvin-statement false The decision between possibility I and IV is made by experimental experience

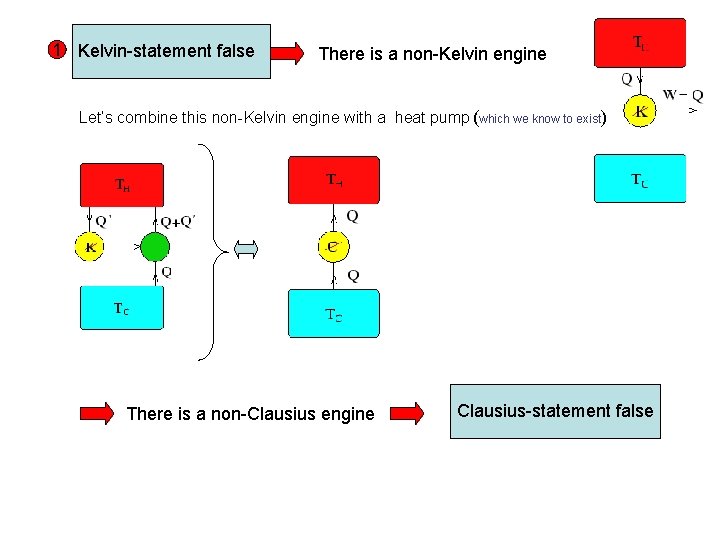
1 Kelvin-statement false There is a non-Kelvin engine Let’s combine this non-Kelvin engine with a heat pump (which we know to exist) There is a non-Clausius engine Clausius-statement false

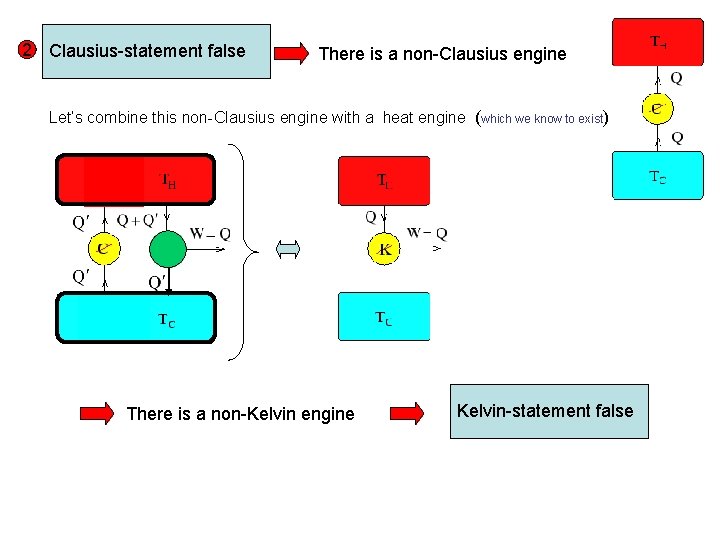
2 Clausius-statement false There is a non-Clausius engine Let’s combine this non-Clausius engine with a heat engine (which we know to exist) There is a non-Kelvin engine Kelvin-statement false
 Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law and second law and third law
Newton's first law and second law and third law Isentropic compressor
Isentropic compressor Zeroth law of thermodynamics
Zeroth law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics 2 nd law of thermodynamics
2 nd law of thermodynamics Define second law of thermodynamics
Define second law of thermodynamics Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law