Second exam Second exam 1 Asample of nitrogen






- Slides: 34

Second exam

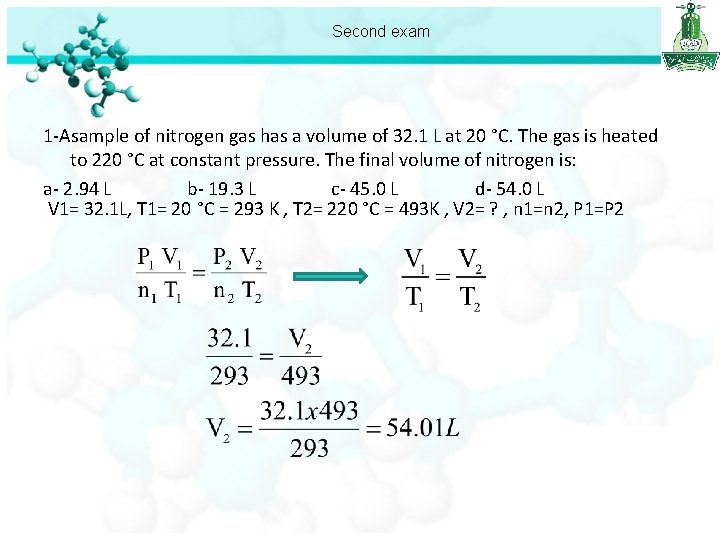
Second exam 1 -Asample of nitrogen gas has a volume of 32. 1 L at 20 °C. The gas is heated to 220 °C at constant pressure. The final volume of nitrogen is: a- 2. 94 L b- 19. 3 L c- 45. 0 L d- 54. 0 L V 1= 32. 1 L, T 1= 20 °C = 293 K , T 2= 220 °C = 493 K , V 2= ? , n 1=n 2, P 1=P 2

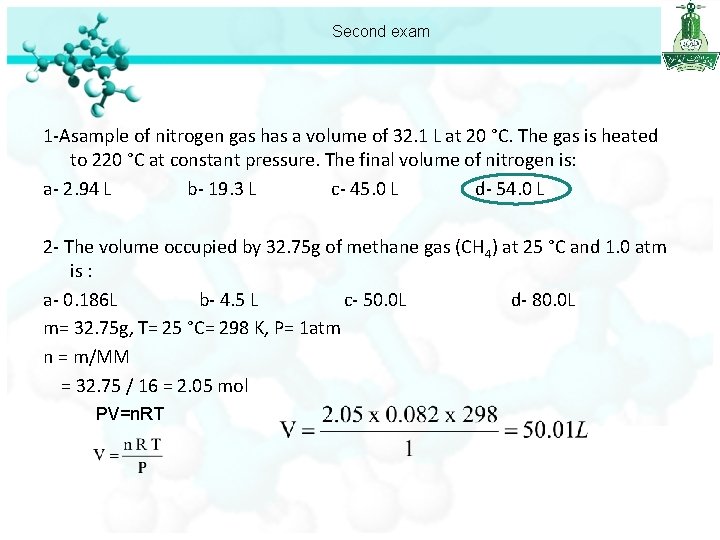
Second exam 1 -Asample of nitrogen gas has a volume of 32. 1 L at 20 °C. The gas is heated to 220 °C at constant pressure. The final volume of nitrogen is: a- 2. 94 L b- 19. 3 L c- 45. 0 L d- 54. 0 L 2 - The volume occupied by 32. 75 g of methane gas (CH 4) at 25 °C and 1. 0 atm is : a- 0. 186 L b- 4. 5 L c- 50. 0 L d- 80. 0 L m= 32. 75 g, T= 25 °C= 298 K, P= 1 atm n = m/MM = 32. 75 / 16 = 2. 05 mol PV=n. RT

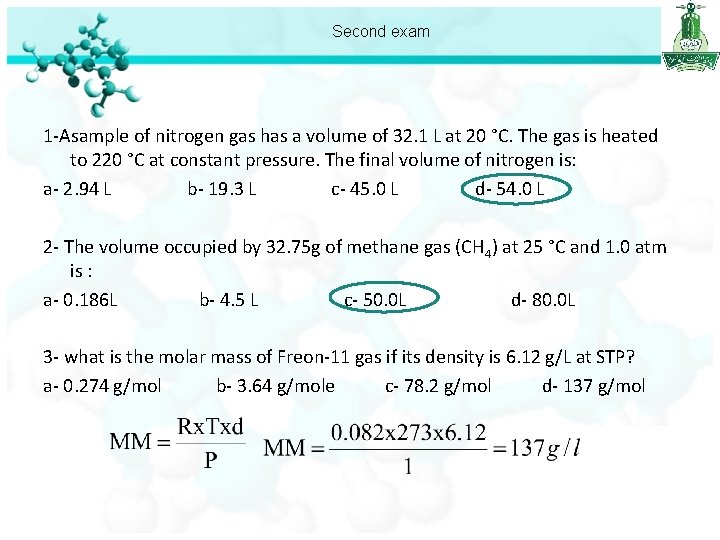
Second exam 1 -Asample of nitrogen gas has a volume of 32. 1 L at 20 °C. The gas is heated to 220 °C at constant pressure. The final volume of nitrogen is: a- 2. 94 L b- 19. 3 L c- 45. 0 L d- 54. 0 L 2 - The volume occupied by 32. 75 g of methane gas (CH 4) at 25 °C and 1. 0 atm is : a- 0. 186 L b- 4. 5 L c- 50. 0 L d- 80. 0 L 3 - what is the molar mass of Freon-11 gas if its density is 6. 12 g/L at STP? a- 0. 274 g/mol b- 3. 64 g/mole c- 78. 2 g/mol d- 137 g/mol

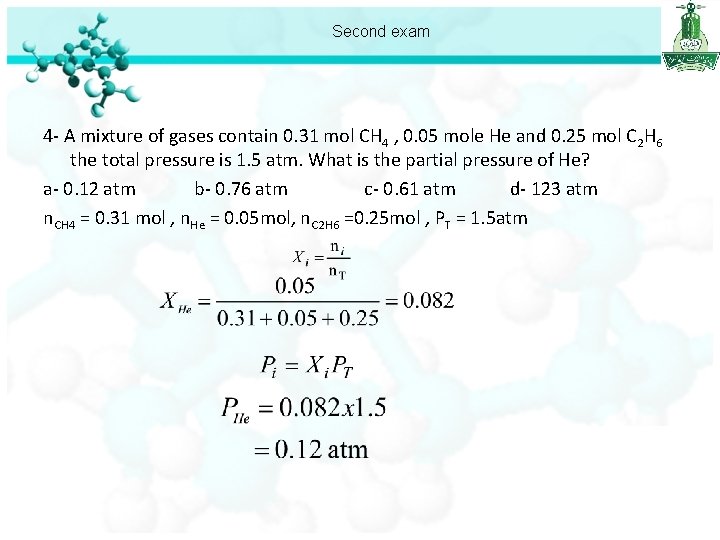
Second exam 1 -Asample of nitrogen gas has a volume of 32. 1 L at 20 °C. The gas is heated to 220 °C at constant pressure. The final volume of nitrogen is: a- 2. 94 L b- 19. 3 L c- 45. 0 L d- 54. 0 L 2 - The volume occupied by 32. 75 g of methane gas (CH 4) at 25 °C and 1. 0 atm is : a- 0. 186 L b- 4. 5 L c- 50. 0 L d- 80. 0 L 3 - what is the molar mass of Freon-11 gas if its density is 6. 12 g/L at STP? a- 0. 274 g/mol b- 3. 64 g/mole c- 78. 2 g/mol d- 137 f/mol 4 - A mixture of gases contain 0. 31 mol CH 4 , 0. 05 mole He and 0. 25 mol 3 C 2 H 6 the total pressure is 1. 5 atm. What is the partial pressure of He? a- 0. 12 atm b- 0. 76 atm c- 0. 61 atm d- 123 atm

Second exam 4 - A mixture of gases contain 0. 31 mol CH 4 , 0. 05 mole He and 0. 25 mol C 2 H 6 the total pressure is 1. 5 atm. What is the partial pressure of He? a- 0. 12 atm b- 0. 76 atm c- 0. 61 atm d- 123 atm n. CH 4 = 0. 31 mol , n. He = 0. 05 mol, n. C 2 H 6 =0. 25 mol , PT = 1. 5 atm

Second exam 1 -Asample of nitrogen gas has a volume of 32. 1 L at 20 °C. The gas is heated to 220 °C at constant pressure. The final volume of nitrogen is: a- 2. 94 L b- 19. 3 L c- 45. 0 L d- 54. 0 L 2 - The volume occupied by 32. 75 g of methane gas (CH 4) at 25 °C and 1. 0 atm is : a- 0. 186 L b- 4. 5 L c- 50. 0 L d- 80. 0 L 3 - what is the molar mass of Freon-11 gas if its density is 6. 12 g/L at STP? a- 0. 274 g/mol b- 3. 64 g/mole c- 78. 2 g/mol d- 137 f/mol 4 - A mixture of gases contain 0. 31 mol CH 4 , 0. 05 mole He and 0. 25 mol 3 C 2 H 6 the total pressure is 1. 5 atm. What is the partial pressure of He? a- 0. 12 atm b- 0. 76 atm c- 0. 61 atm d- 123 atm

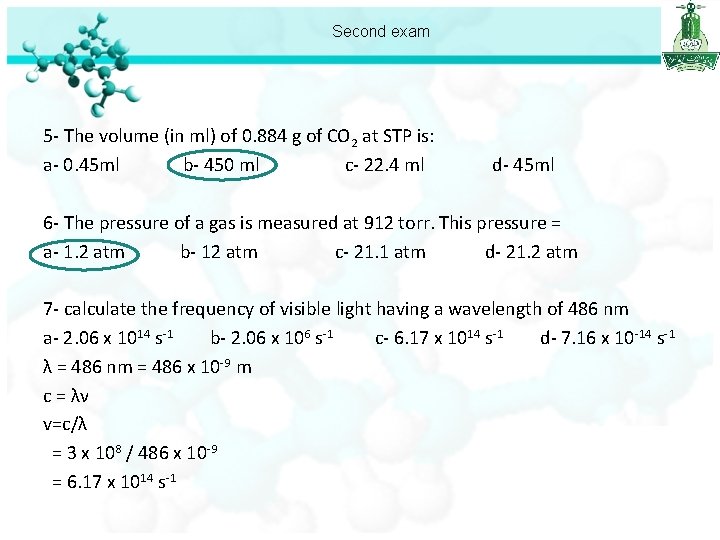
Second exam 5 - The volume (in ml) of 0. 884 g of CO 2 at STP is: a- 0. 45 ml b- 450 ml c- 22. 4 ml m = 0. 884 g, T=273 K, P= 1 atm n= m/MM = 0. 884 / 44 =0. 02 mol PV=n. RT d- 45 ml

Second exam 5 - The volume (in ml) of 0. 884 g of CO 2 at STP is: a- 0. 45 ml b- 450 ml c- 22. 4 ml d- 45 ml 6 - The pressure of a gas is measured at 912 torr. This pressure = a- 1. 2 atm b- 12 atm c- 21. 1 atm d- 21. 2 atm 1 atm == 760 torr ? atm == 912 torr 912 /760 = 1. 2 atm

Second exam 5 - The volume (in ml) of 0. 884 g of CO 2 at STP is: a- 0. 45 ml b- 450 ml c- 22. 4 ml d- 45 ml 6 - The pressure of a gas is measured at 912 torr. This pressure = a- 1. 2 atm b- 12 atm c- 21. 1 atm d- 21. 2 atm 7 - calculate the frequency of visible light having a wavelength of 486 nm a- 2. 06 x 1014 s-1 b- 2. 06 x 106 s-1 c- 6. 17 x 1014 s-1 d- 7. 16 x 10 -14 s-1 λ = 486 nm = 486 x 10 -9 m c = λν v=c/λ = 3 x 108 / 486 x 10 -9 = 6. 17 x 1014 s-1

Second exam 5 - The volume (in ml) of 0. 884 g of CO 2 at STP is: a- 0. 45 ml b- 450 ml c- 22. 4 ml d- 45 ml 6 - The pressure of a gas is measured at 912 torr. This pressure = a- 1. 2 atm b- 12 atm c- 21. 1 atm d- 21. 2 atm 7 - calculate the frequency of visible light having a wavelength of 486 nm a- 2. 06 x 1014 s-1 b- 2. 06 x 106 s-1 c- 6. 17 x 1014 s-1 d- 7. 16 x 10 -14 s-1 8 - Complete this sentence: Atoms emit visible and ultraviolet light : a- as electrons transfer from lower energy levels to higher levels. b- as electrons transfer from higher energy levels to lower levels. c- as they heated and the solid melts to form liquid. d- as electrons move within an orbit.

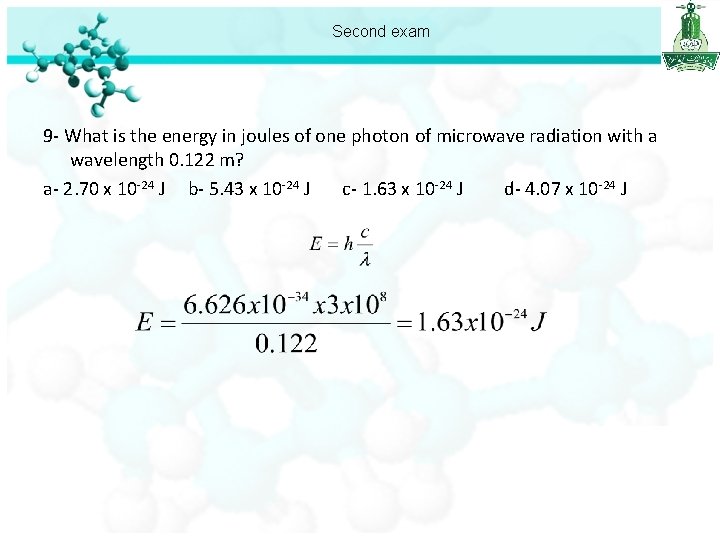
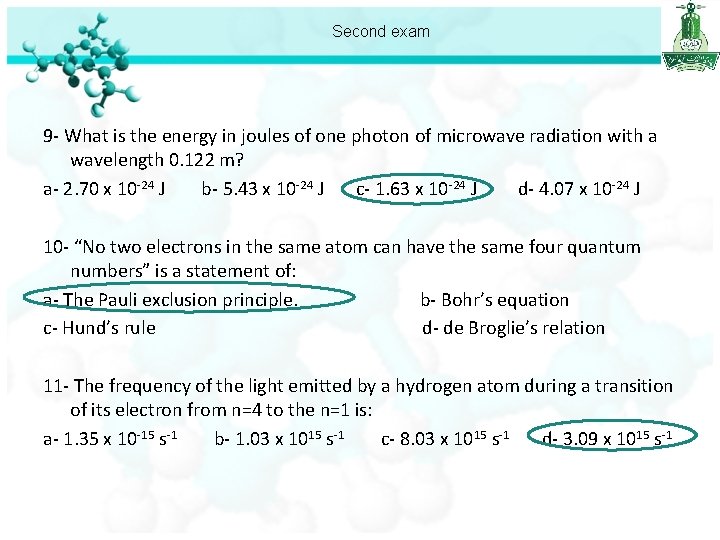
Second exam 9 - What is the energy in joules of one photon of microwave radiation with a wavelength 0. 122 m? a- 2. 70 x 10 -24 J b- 5. 43 x 10 -24 J c- 1. 63 x 10 -24 J d- 4. 07 x 10 -24 J

Second exam 9 - What is the energy in joules of one photon of microwave radiation with a wavelength 0. 122 m? a- 2. 70 x 10 -24 J b- 5. 43 x 10 -24 J c- 1. 63 x 10 -24 J d- 4. 07 x 10 -24 J 10 - “No two electrons in the same atom can have the same four quantum numbers” is a statement of: a- The Pauli exclusion principle. b- Bohr’s equation c- Hund’s rule d- de Broglie’s relation 11 - The frequency of the light emitted by a hydrogen atom during a transition of its electron from n=4 to the n=1 is: a- 1. 35 x 10 -15 s-1 b- 1. 03 x 1015 s-1 c- 8. 03 x 1015 s-1 d- 3. 09 x 1015 s-1

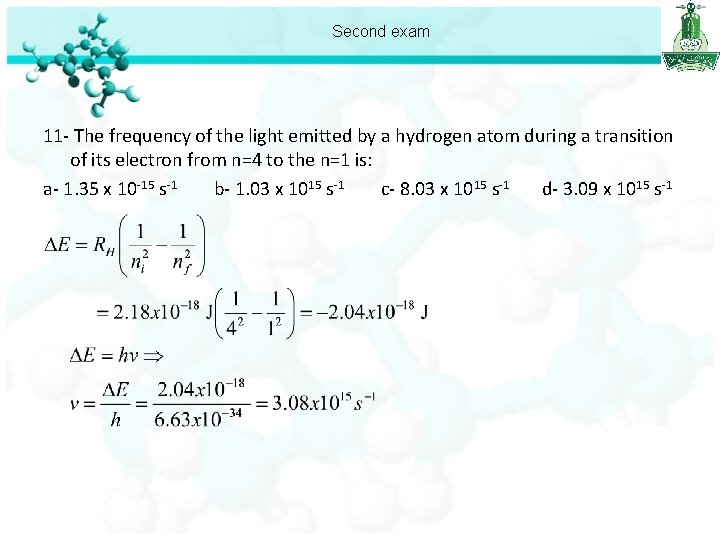
Second exam 11 - The frequency of the light emitted by a hydrogen atom during a transition of its electron from n=4 to the n=1 is: a- 1. 35 x 10 -15 s-1 b- 1. 03 x 1015 s-1 c- 8. 03 x 1015 s-1 d- 3. 09 x 1015 s-1

Second exam 9 - What is the energy in joules of one photon of microwave radiation with a wavelength 0. 122 m? a- 2. 70 x 10 -24 J b- 5. 43 x 10 -24 J c- 1. 63 x 10 -24 J d- 4. 07 x 10 -24 J 10 - “No two electrons in the same atom can have the same four quantum numbers” is a statement of: a- The Pauli exclusion principle. b- Bohr’s equation c- Hund’s rule d- de Broglie’s relation 11 - The frequency of the light emitted by a hydrogen atom during a transition of its electron from n=4 to the n=1 is: a- 1. 35 x 10 -15 s-1 b- 1. 03 x 1015 s-1 c- 8. 03 x 1015 s-1 d- 3. 09 x 1015 s-1

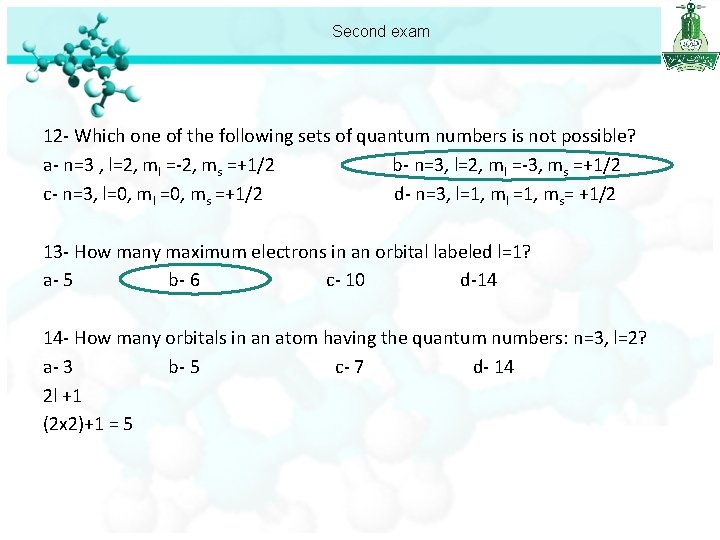
Second exam 12 - Which one of the following sets of quantum numbers is not possible? a- n=3 , l=2, ml =-2, ms =+1/2 b- n=3, l=2, ml =-3, ms =+1/2 c- n=3, l=0, ml =0, ms =+1/2 d- n=3, l=1, ml =1, ms= +1/2 13 - How many maximum electrons in an orbital labeled l=1? a- 5 b- 6 c- 10 d-14 L=1 =p

Second exam 12 - Which one of the following sets of quantum numbers is not possible? a- n=3 , l=2, ml =-2, ms =+1/2 b- n=3, l=2, ml =-3, ms =+1/2 c- n=3, l=0, ml =0, ms =+1/2 d- n=3, l=1, ml =1, ms= +1/2 13 - How many maximum electrons in an orbital labeled l=1? a- 5 b- 6 c- 10 d-14 14 - How many orbitals in an atom having the quantum numbers: n=3, l=2? a- 3 b- 5 c- 7 d- 14 2 l +1 (2 x 2)+1 = 5

Second exam 12 - Which one of the following sets of quantum numbers is not possible? a- n=3 , l=2, ml =-2, ms =+1/2 b- n=3, l=2, ml =-3, ms =+1/2 c- n=3, l=0, ml =0, ms =+1/2 d- n=3, l=1, ml =1, ms= +1/2 13 - How many maximum electrons in an orbital labeled l=1? a- 5 b- 6 c- 10 d-14 14 - How many orbitals in an atom having the quantum numbers: n=3, l=2? a- 3 b- 5 c- 7 d- 14 15 -How many unpaired electrons dose a ground-state atom of sulfur have? a- 0 b- 1 c- 2 d- 3 S=16 e 1 s 2 2 p 6 3 s 2 3 p 4 2 4 3 s 3 p

Second exam 12 - Which one of the following sets of quantum numbers is not possible? a- n=3 , l=2, ml =-2, ms =+1/2 b- n=3, l=2, ml =-3, ms =+1/2 c- n=3, l=0, ml =0, ms =+1/2 d- n=3, l=1, ml =1, ms= +1/2 13 - How many maximum electrons in an orbital labeled l=1? a- 5 b- 6 c- 10 d-14 14 - How many orbitals in an atom having the quantum numbers: n=3, l=2? a- 3 b- 5 c- 7 d- 14 15 -How many unpaired electrons dose a ground-state atom of sulfur have? a- 0 b- 1 c- 2 d- 3

Second exam 16 - The electron configuration of S-2 is : a- 1 s 2 2 p 6 3 s 2 3 p 4 c- 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 2 S-2 = 18 e 1 s 2 2 p 6 3 s 2 3 p 6 b- 1 s 2 2 p 6 3 s 2 3 p 6 d- 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6

Second exam 16 - The electron configuration of S-2 is : a- 1 s 2 2 p 6 3 s 2 3 p 4 c- 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 2 b- 1 s 2 2 p 6 3 s 2 3 p 6 d- 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 17 - The elements in group 2 A are known by what name? a- Transition metals b- Halogen c- Alkali metals d- Alkali earth metals 18 - Which one of these elements is a transition element? a- Nickel b- Tin c- Sodium d- Sulfur 19 - The general electron configuration for atoms of all elements in group 5 A is a- ns 2 nd 3 b- ns 2 np 5 c- ns 2 np 4 d- ns 2 np 3

Second exam 20 - In what group of the periodic table is the element with the elecgron configuration : [Ar]4 s 23 d 104 p 3? a- 2 A b- 3 A c- 5 A d- 7 A Ar=18 e 18+2+10+3 =33 e= As

Second exam 20 - In what group of the periodic table is the element with the electron configuration : [Ar]4 s 23 d 104 p 3? a- 2 A b- 3 A c- 5 A d- 7 A 21 - In which of these pairs of atoms would the bond be the most polar? a- C-C b- C-N c- C-O d- C-S

Second exam 20 - In what group of the periodic table is the element with the electron configuration : [Ar]4 s 23 d 104 p 3? a- 2 A b- 3 A c- 5 A d- 7 A 21 - In which of these pairs of atoms would the bond be the most polar? a- C-C b- C-N c- C-O d- C-S 22 - Which ion is isoelectronic with Ar? a- Fe+2 b- Fc- Br. Ar = 18 e Fe+2 =24 e, F- = 10 e, Br- = 36 e, Ca+2 = 18 e d- Ca+2

Second exam 20 - In what group of the periodic table is the element with the electron configuration : [Ar]4 s 23 d 104 p 3? a- 2 A b- 3 A c- 5 A d- 7 A 21 - In which of these pairs of atoms would the bond be the most polar? a- C-C b- C-N c- C-O d- C-S 22 - Which ion is isoelectronic with Ar? a- Fe+2 b- Fc- Br- d- Ca+2 23 - Which of these choices is the electron configuration of the iron (III) ion? a- [Ar] 3 d 5 b-[Ar] 4 s 1 3 d 5 c- [Ar] 4 s 2 3 d 3 d- [Ar] 3 d 6 Fe = 26 e = [Ar] 4 s 2 3 d 6 Fe+3 = 23 e= [Ar] 3 d 5

Second exam 20 - In what group of the periodic table is the element with the electron configuration : [Ar]4 s 23 d 104 p 3? a- 2 A b- 3 A c- 5 A d- 7 A 21 - In which of these pairs of atoms would the bond be the most polar? a- C-C b- C-N c- C-O d- C-S 22 - Which ion is isoelectronic with Ar? a- Fe+2 b- Fc- Br- d- Ca+2 23 - Which of these choices is the electron configuration of the iron (III) ion? a- [Ar] 3 d 5 b-[Ar] 4 s 1 3 d 5 c- [Ar] 4 s 2 3 d 3 d- [Ar] 3 d 6

Second exam 24 - Which one of these atoms has the largest atomic radius: K, P, S, Cl a- P b- K c- Cl d- S 25 - Which of these compound is most likely to be covalent? a- Rb 2 S b- Cu. Cl 2 c- CS 2 d- Ca. O 26 - The de Broglie wavelength of a ball which has a mass of 250 g and traveling with a speed of 32. 9 m/s is : a- 8. 1 x 1033 m b- 8. 1 x 10 -35 m c- 8. 1 x 10 -33 m d- 8. 1 x 1035 m m =250 g = 0. 25 kg , u= 32. 9 m/s

Second exam 24 - Which one of these atoms has the largest atomic radius: K, P, S, Cl a- P b- K c- Cl d- S 25 - Which of these compound is most likely to be covalent? a- Rb 2 S b- Cu. Cl 2 c- CS 2 d- Ca. O 26 - The de Broglie wavelength of a ball which has a mass of 250 g and traveling with a speed of 32. 9 m/s is : a- 8. 1 x 1033 m b- 8. 1 x 10 -35 m c- 8. 1 x 10 -33 m d- 8. 1 x 1035 m 27 - How many total valance electrons are available in PO 4 -3 ? a- 18 b- 32 c- 29 d- 30 P= 5, O=6, charge =+3 5+(4 X 6)+3 =32

Second exam 24 - Which one of these atoms has the largest atomic radius: K, P, S, Cl a- P b- K c- Cl d- S 25 - Which of these compound is most likely to be covalent? a- Rb 2 S b- Cu. Cl 2 c- CS 2 d- Ca. O 26 - The de Broglie wavelength of a ball which has a mass of 250 g and traveling with a speed of 32. 9 m/s is : a- 8. 1 x 1033 m b- 8. 1 x 10 -35 m c- 8. 1 x 10 -33 m d- 8. 1 x 1035 m 27 - How many total valance electrons are available in PO 4 -3 ? a- 18 b- 32 c- 29 d- 30

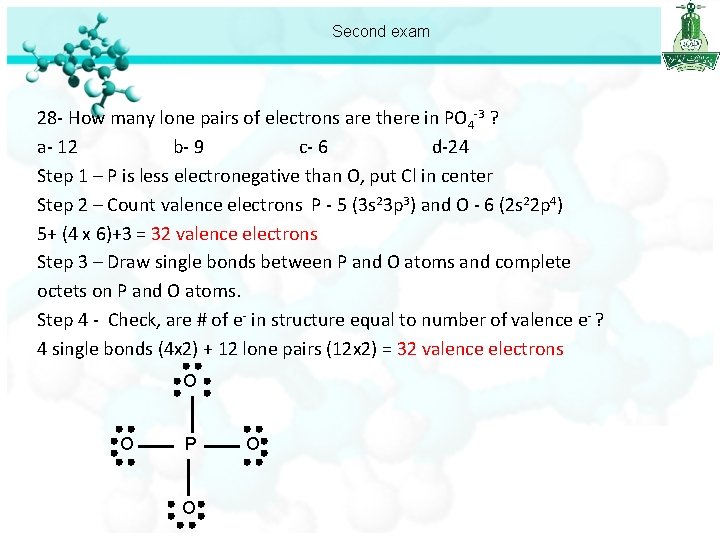
Second exam 28 - How many lone pairs of electrons are there in PO 4 -3 ? a- 12 b- 9 c- 6 d-24 Step 1 – P is less electronegative than O, put Cl in center Step 2 – Count valence electrons P - 5 (3 s 23 p 3) and O - 6 (2 s 22 p 4) 5+ (4 x 6)+3 = 32 valence electrons Step 3 – Draw single bonds between P and O atoms and complete octets on P and O atoms. Step 4 - Check, are # of e- in structure equal to number of valence e- ? 4 single bonds (4 x 2) + 12 lone pairs (12 x 2) = 32 valence electrons O O P O O

Second exam 28 - How many lone pairs of electrons are there in PO 4 -3 ? a- 12 b- 9 c- 6 d-24 29 - How many resonance structures are there in PO 4 -3 ? a- 0 b- 4 c- 2 d- 3 30 - The formal charge on the phosphorus atom in PO 4 -3 ? a- 0 b- +2 c- (-1) d- +1

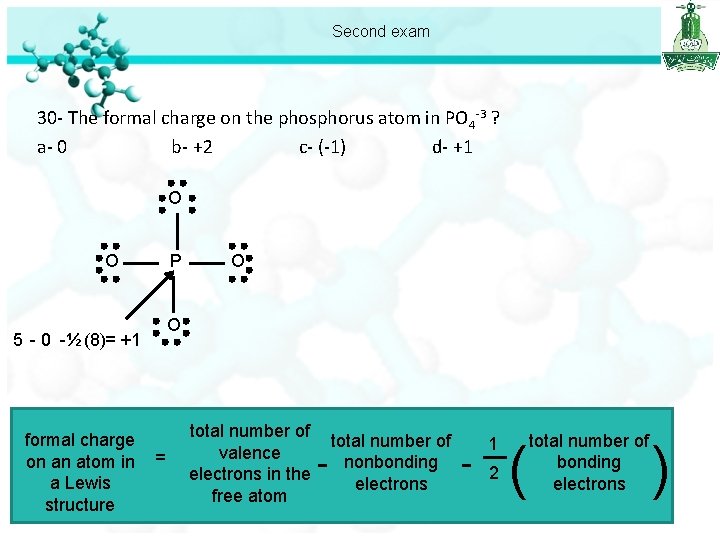
Second exam 30 - The formal charge on the phosphorus atom in PO 4 -3 ? a- 0 b- +2 c- (-1) d- +1 O O P O 5 - 0 -½ (8)= +1 formal charge on an atom in a Lewis structure O = total number of valence electrons in the free atom - total number of nonbonding electrons - 1 2 ( total number of bonding electrons )

Second exam 28 - How many lone pairs of electrons are there in PO 4 -3 ? a- 12 b- 9 c- 6 d-24 29 - How many resonance structures are there in PO 4 -3 ? a- 0 b- 4 c- 2 d- 3 30 - The formal charge on the phosphorus atom in PO 4 -3 ? a- 0 b- +2 c- (-1) d- +1
