Screening for hypotyreosis and congenital adrenal hyperplasia CAH



- Slides: 34

Screening for hypotyreosis and congenital adrenal hyperplasia (CAH), second-tier testing – laboratory methods Péter Monostori

Definitions of positive/negative predictive value, sensitivity and specificity ØSensitivity Ø The proportion of affected subjects that have a positive test result ØSpecificity Ø The proportion of unaffected subjects that have a negative test result ØPositive predictive value Ø The chance that a positive test result actually indicates an a�ected individual Ø The proportion of „real” positive samples within all positive results ØNegative predictive value Ø The chance that a negative test result actually excludes the disorder Ø The proportion of „real” negative samples within all negative results

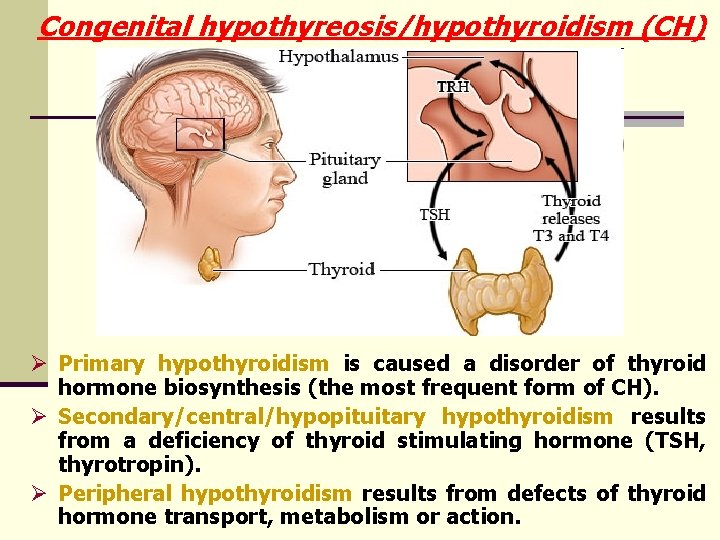
Congenital hypothyreosis/hypothyroidism (CH) Ø Primary hypothyroidism is caused a disorder of thyroid hormone biosynthesis (the most frequent form of CH). Ø Secondary/central/hypopituitary hypothyroidism results from a deficiency of thyroid stimulating hormone (TSH, thyrotropin). Ø Peripheral hypothyroidism results from defects of thyroid hormone transport, metabolism or action.

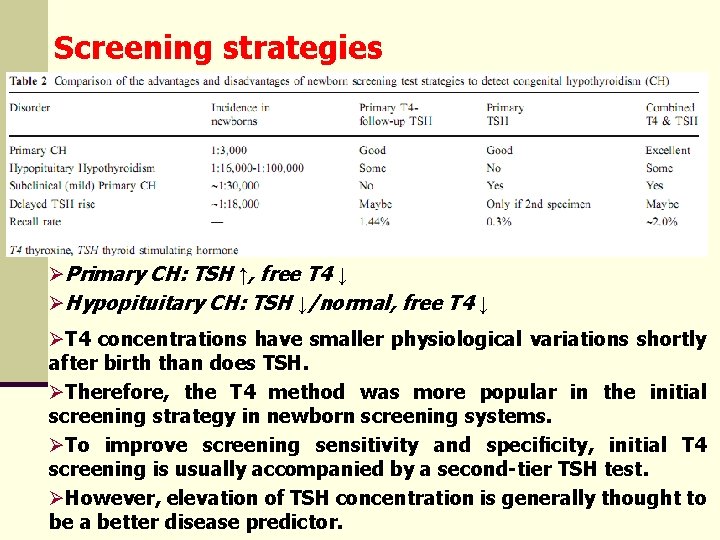
Screening strategies ØPrimary CH: TSH ↑, free T 4 ↓ ØHypopituitary CH: TSH ↓/normal, free T 4 ↓ ØT 4 concentrations have smaller physiological variations shortly after birth than does TSH. ØTherefore, the T 4 method was more popular in the initial screening strategy in newborn screening systems. ØTo improve screening sensitivity and specificity, initial T 4 screening is usually accompanied by a second-tier TSH test. ØHowever, elevation of TSH concentration is generally thought to be a better disease predictor.

Screening methodology ØT 4 screening Ø Radioimmunoassay (RIA): Øradioactively labeled specific antibody and an immobilized antibody are used Øthis method was associated with lower CH incidences than the other techniques Øis rarely used nowadays Ø Enzyme immunometric assay (EIA): an enzyme is connected to a specific antibody Ø Fluoroimmunoassay (FIA): a fluorophore is connected to a specific antibody ØPrimary T 4+follow-up TSH test: Ø Advantage: infants with hypopituitary hypothyroidism and delayed TSH rise may also be detected. Ø Disadvantage: lower sensitivity and specificity, higher recall rates.

Screening methodology ØTSH screening Ø RIA: Øthis method was associated with lower CH incidences than the other techniques Øis rarely used nowadays Ø Immunoradiometric assay (IRMA): „improved RIA”, radioactively labeled specific antibody and an immobilized antibody are used Ø EIA Ø FIA, incl. dissociation-enhanced, lanthanide fluorescence immunoassay (DELFIA®) ØPrimary TSH test: Ø Advantage: cases of mild subclinical hypothyroidism are also detected, and lower recall rates are needed. If a second routine sample is obtained, infants with delayed TSH rise are also detected. Ø Disadvantage: infants with hypopituitary hypothyroidism cannot be detected.

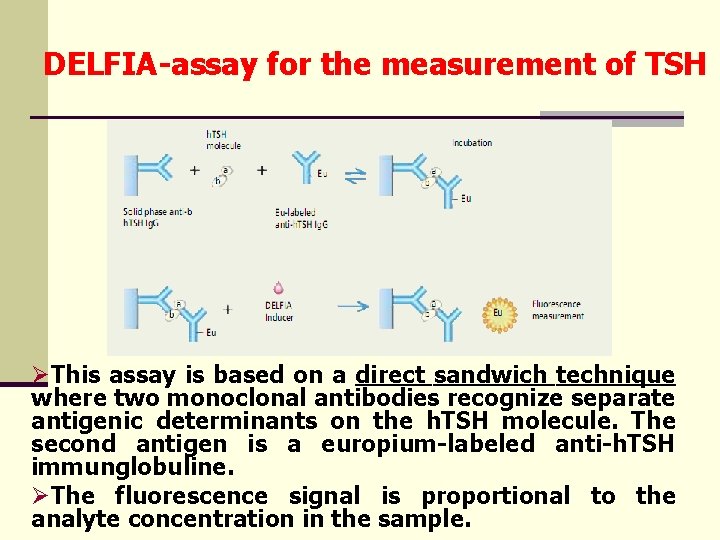
DELFIA-assay for the measurement of TSH ØThis assay is based on a direct sandwich technique where two monoclonal antibodies recognize separate antigenic determinants on the h. TSH molecule. The second antigen is a europium-labeled anti-h. TSH immunglobuline. ØThe fluorescence signal is proportional to the analyte concentration in the sample.

DELFIA-assay for hypothyreosis ØQuantitative ØReproducible ØSensitivity: 2 μU/ml ØIncubation time: 4 h, or overnight ØEDTA (such as in violet-capped tubes) disturbs the determination (chelates europium)

Screening methodology ØCombined T 4 and TSH screening: Ø has the advantages of both previous methods Ø disadvantage: more expensive and laborious, higher recall rates

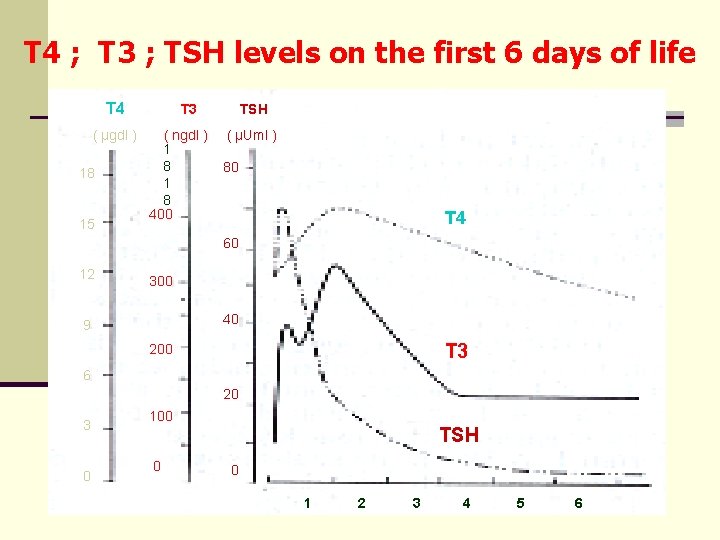
T 4 ; T 3 ; TSH levels on the first 6 days of life T 4 ( μgdl ) 18 15 T 3 ( ngdl ) 1 8 400 TSH ( μUml ) 80 T 4 60 12 300 40 9 T 3 200 6 20 3 0 100 TSH 0 0 1 2 3 4 5 6

The timing of blood sampling for CH screening Øimportant, as T 4, T 3 and TSH levels decrease with age Øfalse-positives: Ø in the first days of life, TSH may be transiently elevated (normally lasting not more than 24 h) Ø premature infants: physiological reduction in TSH may occur Øfalse-negative rate: Ø is higher if T 4 is primarily screened (this is probably associated with the residual thyroid hormone activity of the mother)

Prenatal diagnosis Ø Genetic testing on fetal cells obtained by amniocentesis is a more direct and safer method of diagnosis than fetal cord blood sampling. Ø Measurement of amniotic fluid TSH or thyroid hormone levels are not reliable, sampling of fetal umbilical cord blood is necessary to diagnose fetal hypothyroidism.

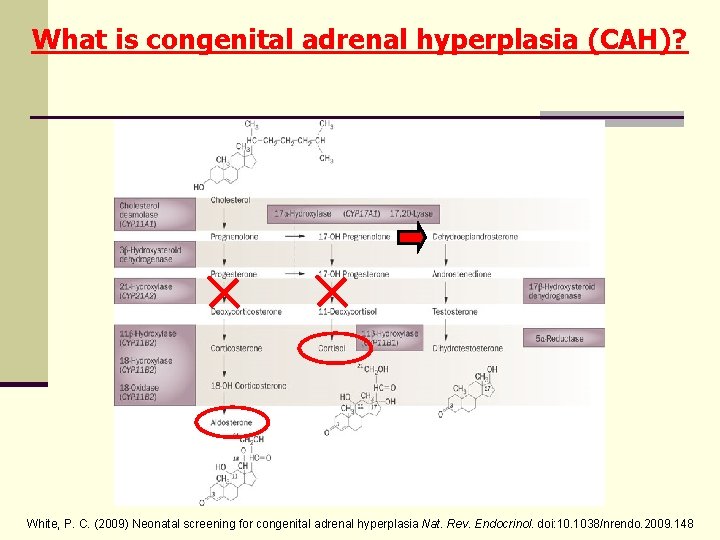
What is congenital adrenal hyperplasia (CAH)? White, P. C. (2009) Neonatal screening for congenital adrenal hyperplasia Nat. Rev. Endocrinol. doi: 10. 1038/nrendo. 2009. 148

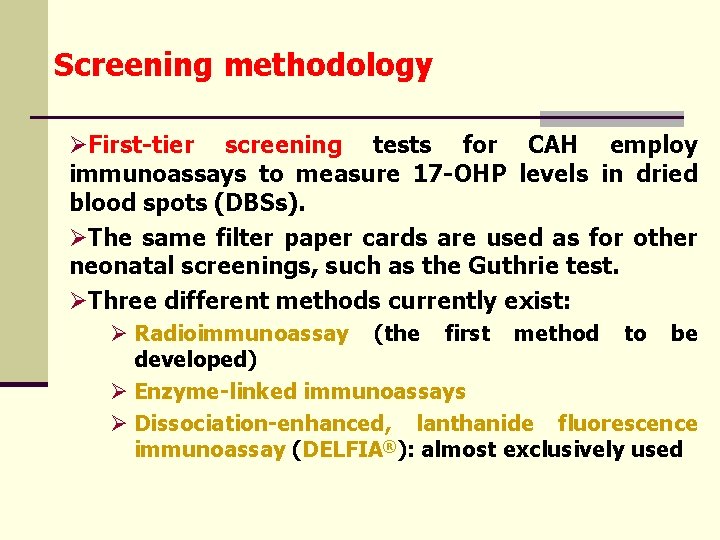
Screening methodology ØFirst-tier screening tests for CAH employ immunoassays to measure 17 -OHP levels in dried blood spots (DBSs). ØThe same filter paper cards are used as for other neonatal screenings, such as the Guthrie test. ØThree different methods currently exist: Ø Radioimmunoassay (the first method to be developed) Ø Enzyme-linked immunoassays Ø Dissociation-enhanced, lanthanide fluorescence immunoassay (DELFIA®): almost exclusively used

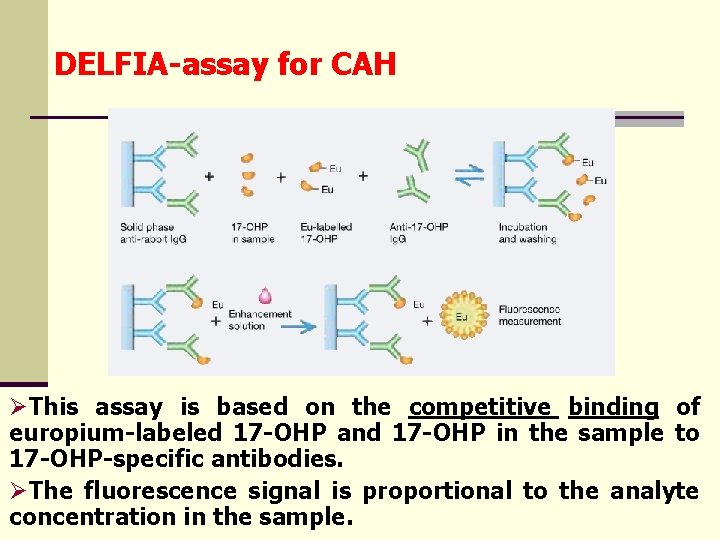
DELFIA-assay for CAH ØThis assay is based on the competitive binding of europium-labeled 17 -OHP and 17 -OHP in the sample to 17 -OHP-specific antibodies. ØThe fluorescence signal is proportional to the analyte concentration in the sample.

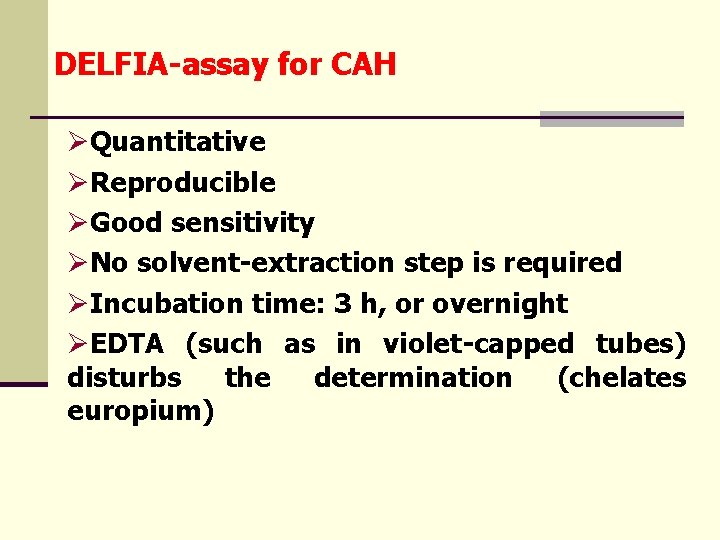
DELFIA-assay for CAH ØQuantitative ØReproducible ØGood sensitivity ØNo solvent-extraction step is required ØIncubation time: 3 h, or overnight ØEDTA (such as in violet-capped tubes) disturbs the determination (chelates europium)

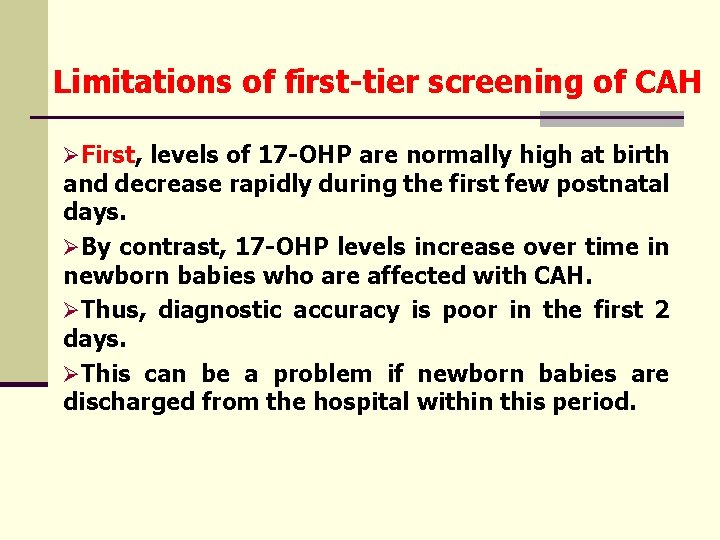
Limitations of first-tier screening of CAH ØFirst, levels of 17 -OHP are normally high at birth and decrease rapidly during the first few postnatal days. ØBy contrast, 17 -OHP levels increase over time in newborn babies who are affected with CAH. ØThus, diagnostic accuracy is poor in the first 2 days. ØThis can be a problem if newborn babies are discharged from the hospital within this period.

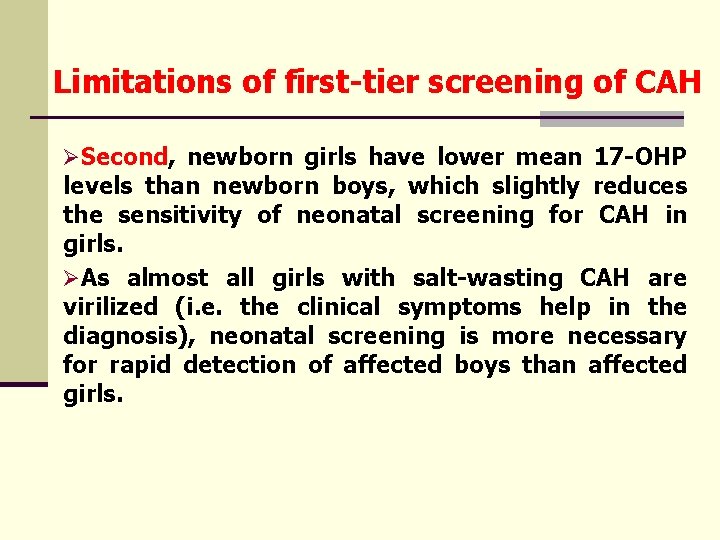
Limitations of first-tier screening of CAH ØSecond, newborn girls have lower mean 17 -OHP levels than newborn boys, which slightly reduces the sensitivity of neonatal screening for CAH in girls. ØAs almost all girls with salt-wasting CAH are virilized (i. e. the clinical symptoms help in the diagnosis), neonatal screening is more necessary for rapid detection of affected boys than affected girls.

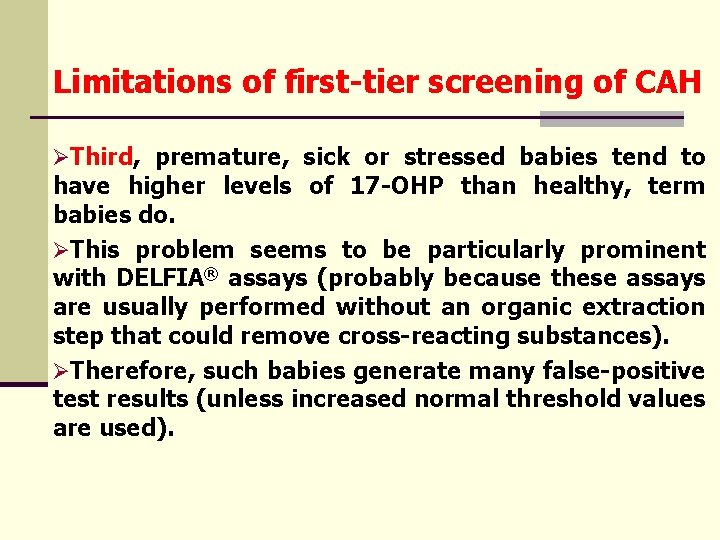
Limitations of first-tier screening of CAH ØThird, premature, sick or stressed babies tend to have higher levels of 17 -OHP than healthy, term babies do. ØThis problem seems to be particularly prominent with DELFIA® assays (probably because these assays are usually performed without an organic extraction step that could remove cross-reacting substances). ØTherefore, such babies generate many false-positive test results (unless increased normal threshold values are used).

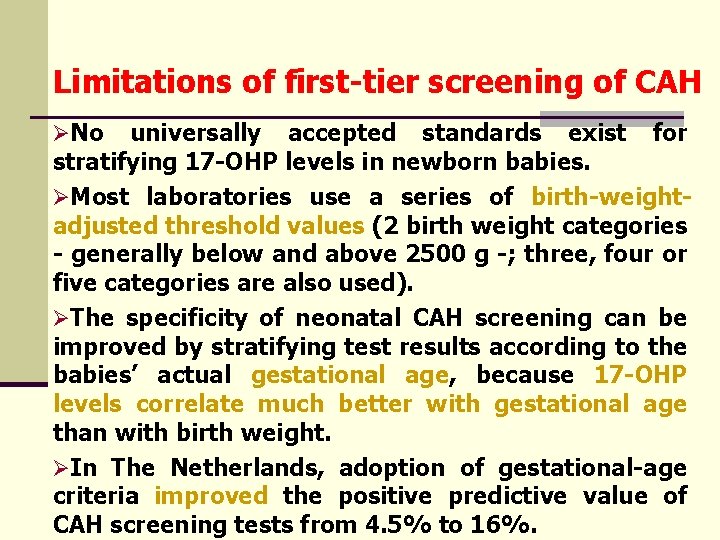
Limitations of first-tier screening of CAH ØNo universally accepted standards exist for stratifying 17 -OHP levels in newborn babies. ØMost laboratories use a series of birth-weightadjusted threshold values (2 birth weight categories - generally below and above 2500 g -; three, four or five categories are also used). ØThe specificity of neonatal CAH screening can be improved by stratifying test results according to the babies’ actual gestational age, because 17 -OHP levels correlate much better with gestational age than with birth weight. ØIn The Netherlands, adoption of gestational-age criteria improved the positive predictive value of CAH screening tests from 4. 5% to 16%.

Limitations of first-tier screening of CAH ØFourth, antenatal corticosteroids administered to mothers at risk of preterm delivery might reduce 17‑OHP levels. ØThis may potentially increase the likelihood of falsenegative screening test results. ØAll babies who received such treatment and were screened for CAH at birth should be tested again after several days of life.

Limitations of first-tier screening of CAH ØFinally, neonatal screening identifies only few babies with mild, nonclassic CAH. ØIncidence: 1: 130000. ØThe true incidence of nonclassic CAH is predicted to be approximately 1: 2000 in most populations (as indicated by the carrier frequency).

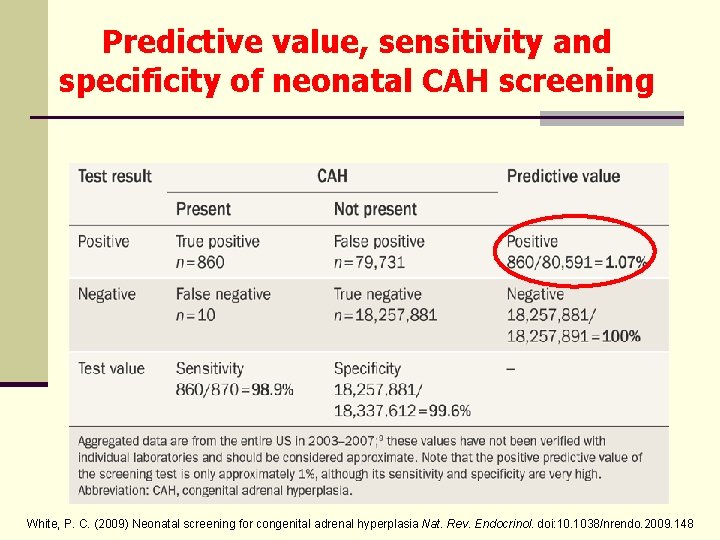
Predictive value, sensitivity and specificity of neonatal CAH screening White, P. C. (2009) Neonatal screening for congenital adrenal hyperplasia Nat. Rev. Endocrinol. doi: 10. 1038/nrendo. 2009. 148

The reason for a need for second-tier screening with CAH ØThe positive predictive value for CAH screening is generally about 1% (despite adjusting cutoff levels for birth weight, gestational age, and stress factors). Ø Organic solvent extraction before immunoassays may be appropriate, but Ødirect biochemical analysis of steroid levels by liquid chromatography-tandem mass spectrometry (LC–MS/MS) addresses these issues more effectively. ØThe run times for individual samples in LC–MS/MS assays are 6– 12 min, which would be too long for a first-tier screen, but are suitable for a second-tier screen using the original DBSs.

Second-tier screening Biochemical second screens ØThe LC-MS/MS assay does not only determine 17 - OHP as a direct substrate for 21 -hydroxylase, but also cortisol (a downstream product of this enzyme’s reaction) and other steroids. ØAppropriately selected ratios of the steroids can further improve the specificity of LC–MS/MS. ØThe rationale for the using ratios with cortisol is that newborns under stress will have high cortisol levels with secondary accumulation of 17 -OHP. ØMeasuring the level of 17 -OHP only will yield elevated results in these cases, which are not related to CAH and do not require follow-up.

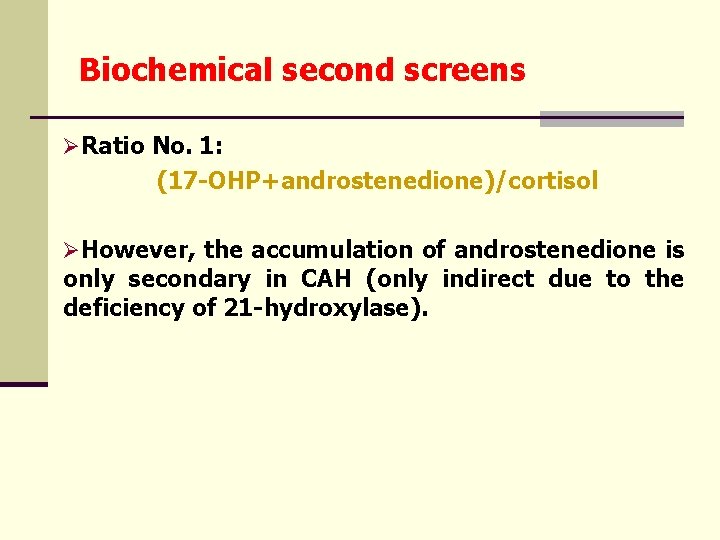
Biochemical second screens ØRatio No. 1: (17 -OHP+androstenedione)/cortisol ØHowever, the accumulation of androstenedione is only secondary in CAH (only indirect due to the deficiency of 21 -hydroxylase).

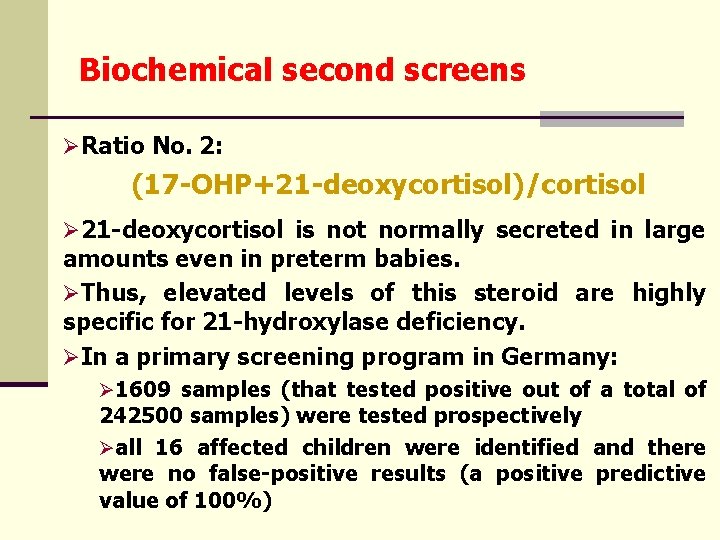
Biochemical second screens ØRatio No. 2: (17 -OHP+21 -deoxycortisol)/cortisol Ø 21 -deoxycortisol is not normally secreted in large amounts even in preterm babies. ØThus, elevated levels of this steroid are highly specific for 21 -hydroxylase deficiency. ØIn a primary screening program in Germany: Ø 1609 samples (that tested positive out of a total of 242500 samples) were tested prospectively Øall 16 affected children were identified and there were no false-positive results (a positive predictive value of 100%)

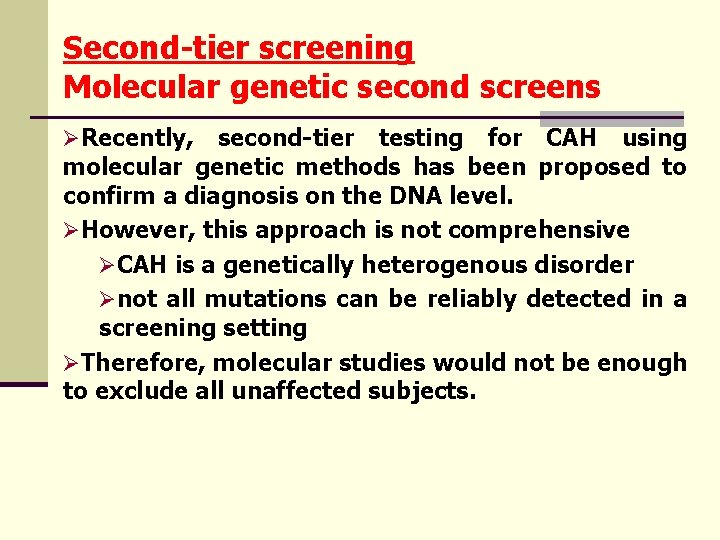
Second-tier screening Molecular genetic second screens ØRecently, second-tier testing for CAH using molecular genetic methods has been proposed to confirm a diagnosis on the DNA level. ØHowever, this approach is not comprehensive ØCAH is a genetically heterogenous disorder Ønot all mutations can be reliably detected in a screening setting ØTherefore, molecular studies would not be enough to exclude all unaffected subjects.

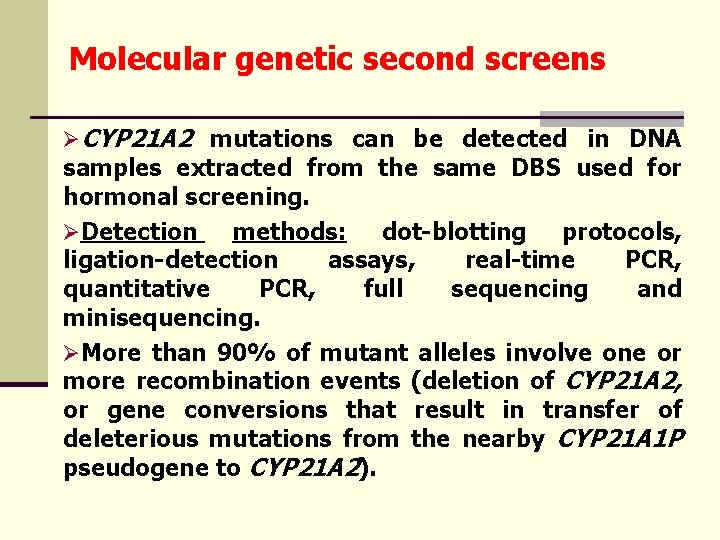
Molecular genetic second screens ØCYP 21 A 2 mutations can be detected in DNA samples extracted from the same DBS used for hormonal screening. ØDetection methods: dot-blotting protocols, ligation-detection assays, real-time PCR, quantitative PCR, full sequencing and minisequencing. ØMore than 90% of mutant alleles involve one or more recombination events (deletion of CYP 21 A 2, or gene conversions that result in transfer of deleterious mutations from the nearby CYP 21 A 1 P pseudogene to CYP 21 A 2).

Second-tier screening Cost-effectiveness ØLC–MS/MS is less costly and time-consuming than genotyping per sample (even equipment for LC–MS/MS is expensive). if the

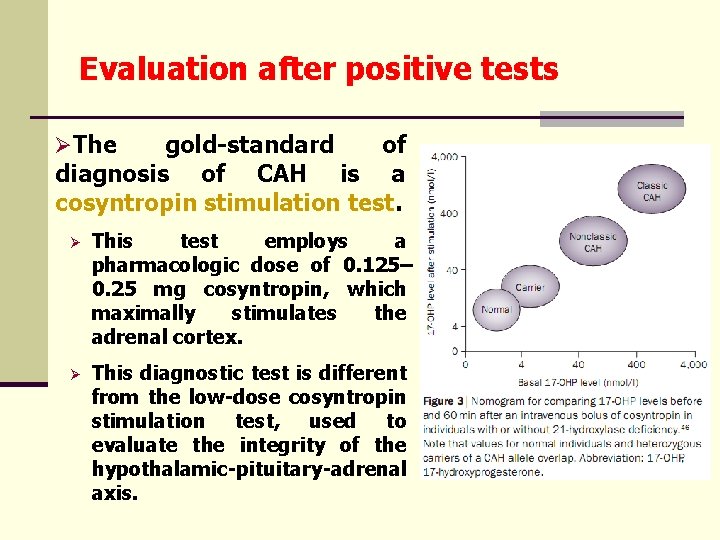
Evaluation after positive tests ØThe gold-standard of diagnosis of CAH is a cosyntropin stimulation test. Ø This test employs a pharmacologic dose of 0. 125– 0. 25 mg cosyntropin, which maximally stimulates the adrenal cortex. Ø This diagnostic test is different from the low-dose cosyntropin stimulation test, used to evaluate the integrity of the hypothalamic-pituitary-adrenal axis.

Evaluation after positive tests/2 ØMeasured analytes stimulation test: during the cosyntropin Ø 17 -OHP, cortisol, deoxycorticosterone, 11 -deoxycortisol and 17 -OH-pregnenolone at 0 min and 60 min of stimulation Ø at least one measurement each of dehydroepiandrosterone (DHEA) androstenedione Ø in low-birth-weight babies who have a small blood volume, only one sample is collected at 60 min Ø determination of precursor: product ratios is particularly useful to distinguish between the different enzymatic defects Ø preferable method: LC-MS/MS

Prenatal diagnosis Ø Samples are obtained by means of chorionic villous sampling at 10 -12 weeks of pregnancy, or by means of amniocentesis at 15 -18 weeks of pregnancy. Ø HLA typing in combination with measurement of 17 -OHP androstenedione/21 -deoxycortisol in amniotic fluid may be used for prenatal diagnosis.

Thank you for your attention!