SCIMATP Presentations CLASSIFICATION OF MATERIALS MR RAYMUND B

SCIMATP Presentations CLASSIFICATION OF MATERIALS MR. RAYMUND B. BOLALIN
Classification of Materials • In Materials Science, classification of materials is usually based on the atomic bonding forces of the materials. • General Classification – Metals – Ceramics – Polymers – Composites – Semiconductors
METALS • Metals are normally combinations of metallic elements. They have large numbers of non-localized electrons. • Properties: 1. shiny luster 2. grayish-silver color (except gold and copper) 3. hardness (except sodium and calcium) 4. good heat and electrical conductivity 5. high melting and boiling points (except mercury) 6. malleability (can be hammered into a sheet) 7. ductility (can be pulled into a wire)

METALS • Examples: • Ferrous metals and alloys (irons, carbon steels, alloy steels, stainless steels, tool and die steels) • Applications: • Nonferrous metals and jewelry, alloys (aluminum, • coinage, automobiles, structural copper, magnesium, components for nickel, titanium, buildings, hulls of large precious metals, ships, etc. refractory metals, Credit: http: //www. ndt-ed. org superalloys)
CERAMICS • Ceramics are compounds between metallic and nonmetallic elements. They are most frequently oxides, nitrides, and carbides and are composed of clay minerals, cement, and glass. • Properties: 1. typically insulative to the passage of heat and electricity 2. more resistant to high temperatures and harsh environments 3. hard but very brittle
CERAMICS • • • Examples Glass ceramics Graphite Diamond • Applications: • insulators, semiconductors, magnets, aerospace, biomedical, construction, nuclear industries Credit: http: //www. ndt-ed. org
POLYMERS • Polymers include the familiar plastics and rubber materials. Many of organic compounds that are based on carbon, hydrogen, nonmetallic elements. They large molecular structures. • Properties: 1. low density 2. may be extremely flexible them are chemically and other have very
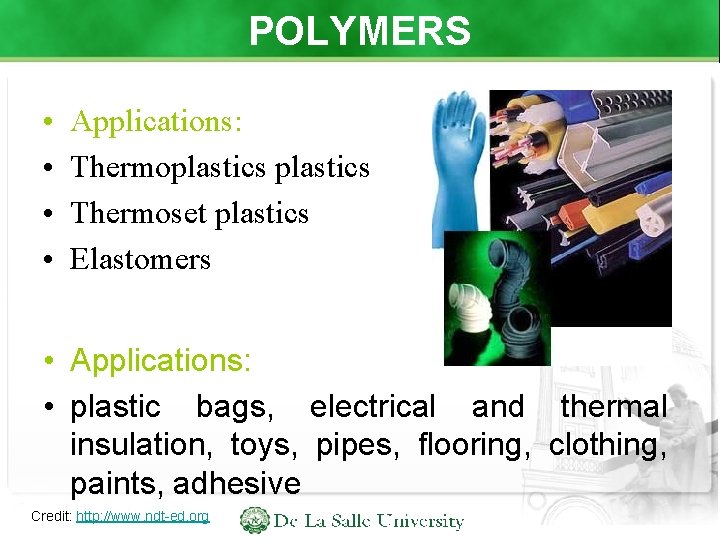
POLYMERS • • Applications: Thermoplastics Thermoset plastics Elastomers • Applications: • plastic bags, electrical and thermal insulation, toys, pipes, flooring, clothing, paints, adhesive Credit: http: //www. ndt-ed. org
COMPOSITES • Composite materials are engineered materials that consist of more than one material type. They are designed to display a combination of the best characteristics of each of the component materials

COMPOSITES • Example: • Fiberglass – glass fibers are embedded within a polymeric material Fiberglass – acquires strength from the glass and flexibility from the polymer • Applications: • space shuttles • construction of pavement, roads, parking lots • armor
SEMICONDUCTORS • Semiconductors are materials with electrical conductivity due to electron flow intermediate in magnitude between that of the electrical conductors and insulators. – Semiconductors form the heart of modern electronics. - The electrical characteristics are extremely sensitive to the presence of minute concentrations of impurity atoms
SEMICONDUCTORS • Examples: – Silicon (Si) – Gallium Phosphide (Ga. P) – Aluminium Gallium Arsenide (Alx. Ga 1 x. As) • Applications: – integrated circuits – electronics – computers Credit: http: //www. ovenind. com
- Slides: 12