SCIENTIFIC NOTATION PERCENT ERROR DERIVED UNITS SPECIFIC GRAVITY






- Slides: 19

SCIENTIFIC NOTATION, PERCENT ERROR, DERIVED UNITS, SPECIFIC GRAVITY SECTION 2. 1 AND 2. 8

SCIENTIFIC NOTATION Rules for placing numbers from standard form into scientific notation: � Write all the significant digits of the numerical portion of the value. Move the decimal point in the numerical portion of the value so there is ONE non-zero digit to the left of the decimal point. � The number of places that the decimal point was moved will be the power, or exponent, of 10 in the exponential portion of the value. � � Moving the decimal to the left is expressed as a positive exponent, and moving the decimal to the right is expressed as a negative exponent.

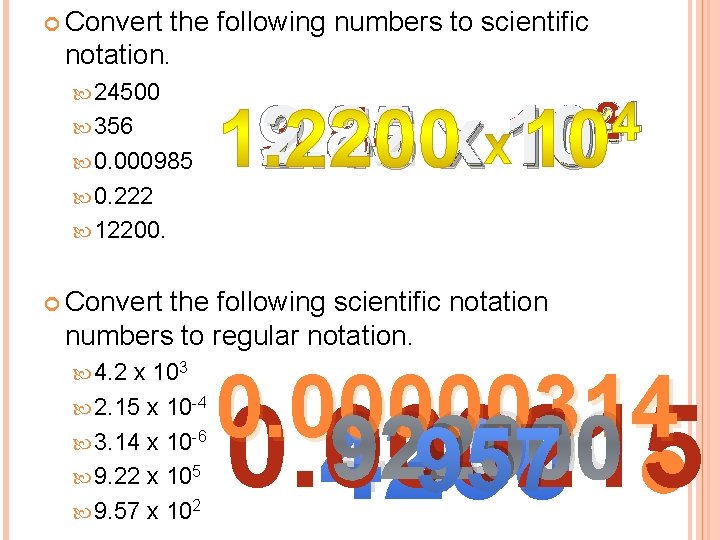
Convert the following numbers to scientific notation. 24500 356 0. 000985 0. 222 2 -1 -4 4 9. 85 x 10 3. 56 x 10 2. 22 x 10 2. 45 x 10 12200. Convert the following scientific notation numbers to regular notation. 4. 2 x 103 2. 15 x 10 -4 3. 14 x 10 -6 9. 22 x 105 9. 57 x 102 0. 00000314 922000 957 0. 000215 4200

RULES FOR CALCULATIONS WITH SCIENTIFIC NOTATION: For addition and subtraction, convert to ordinary notation before performing the operation. This will allow you to correctly count the number or decimal places needed in your final answer. For multiplication and division, your calculator will put the answer into correct scientific notation for you.

ROUNDING OFF: Calculators will often present answers to calculations with many more figures than the significant ones. As a result many of the figures shown are meaningless and the answer, before it is presented, needs to be rounded off. In a series of calculations always leave the rounding off to the end, i. e. leave all numbers on the calculator in the intermediate steps. Use the simple rule that if the digit directly to the right of the final significant figure is less that 5 then the preceding digit stays the same, if it is equal to or greater than 5 then the preceding digit should be increased by one.

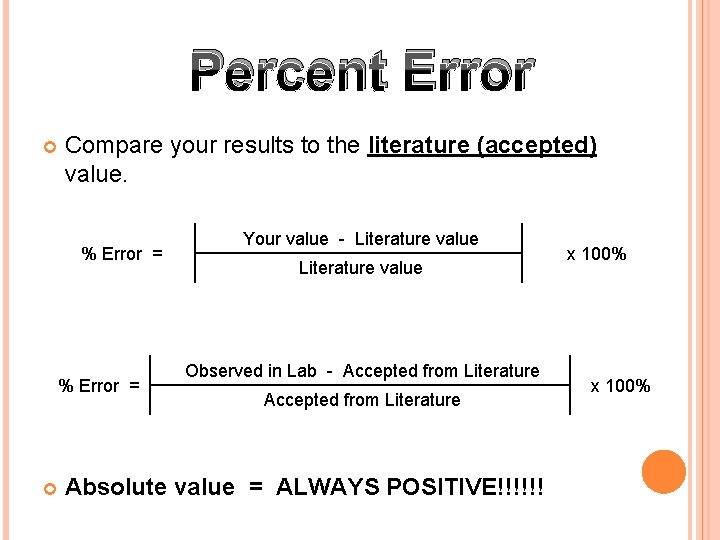
Percent Error Compare your results to the literature (accepted) value. % Error = Your value - Literature value Observed in Lab - Accepted from Literature Absolute value = ALWAYS POSITIVE!!!!!! x 100%

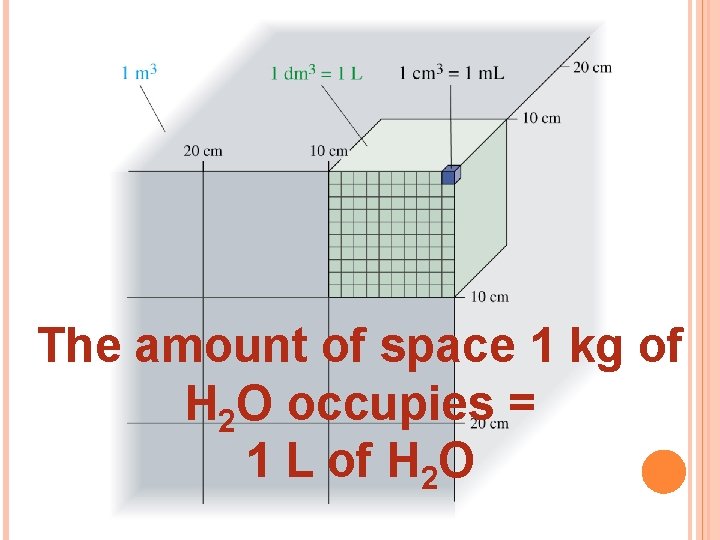
DERIVED UNITS All other units can be derived from base quantities. This combination of base units is called a derived unit. Examples: speed length/time m/s volume (length) m 3 We will use volume measurements a lot, so it is important to remember these relationships: 1 dm 3 = 1 L = 1000 m. L = 1000 cm 3

The amount of space 1 kg of H 2 O occupies = 1 L of H 2 O

QUESTION: Which is heavier a ton of feathers or a ton of lead? Neither, they both have the same weight. The better question would be “Which is more DENSE? ” The lead is more dense.

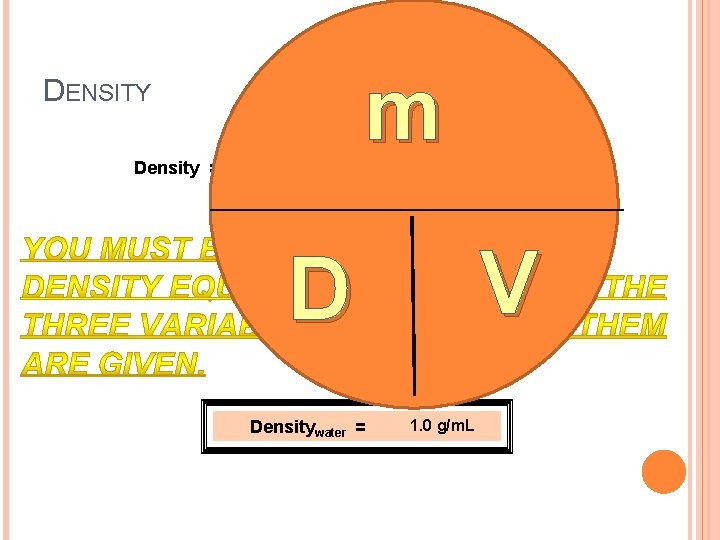
DENSITY Density = mass m volume D Densitywater = = g m. L or cm 3 V 1. 0 g/m. L

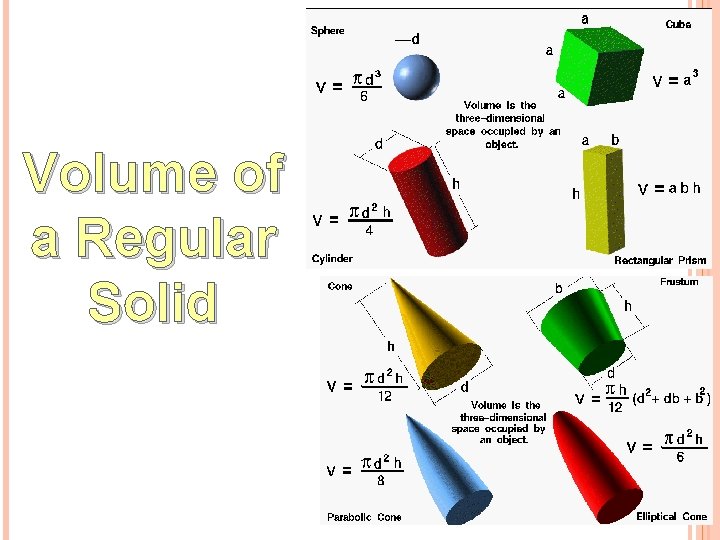
Volume of a Regular Solid

ARCHIMEDES – WATER DISPLACEMENT Hiero II, king of Syracuse (a region in Greece) during the 3 rd century BC; commissioned the production of a gold crown. Suspected that he was being defrauded by substituting a less precious metal in the interior. Hiero asked Archimedes to determine if the crown was made of pure gold. Could not damage or disassemble the crown. Non-destructive testing

I’ve Found Archimedes placed a block of pure gold equal to the mass of the crown in a It!!!! container and filled it to the brim with water. He then removed the gold and placed the crown in the container and noticed that water overflowed. He concluded that the king was cheated because the crown had the same mass as the gold block, but had a larger volume and caused the water to overflow.

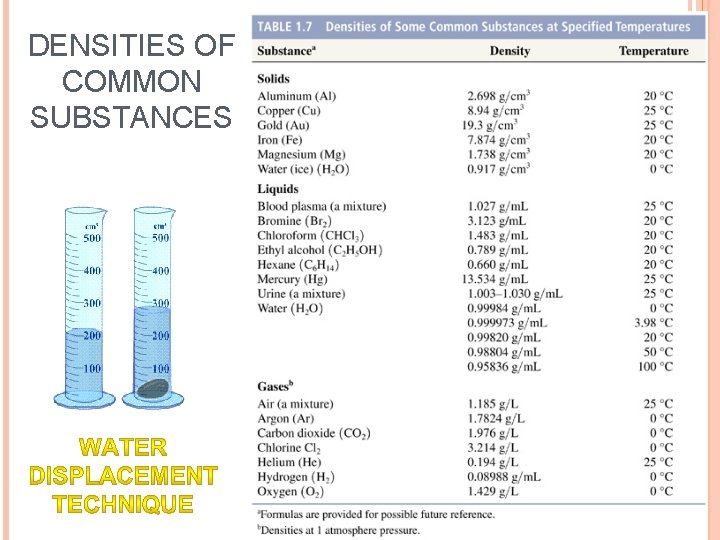
DENSITIES OF COMMON SUBSTANCES

WHAT DETERMINES WHETHER SOMETHING WILL SINK OR FLOAT? density of the object density of the liquid

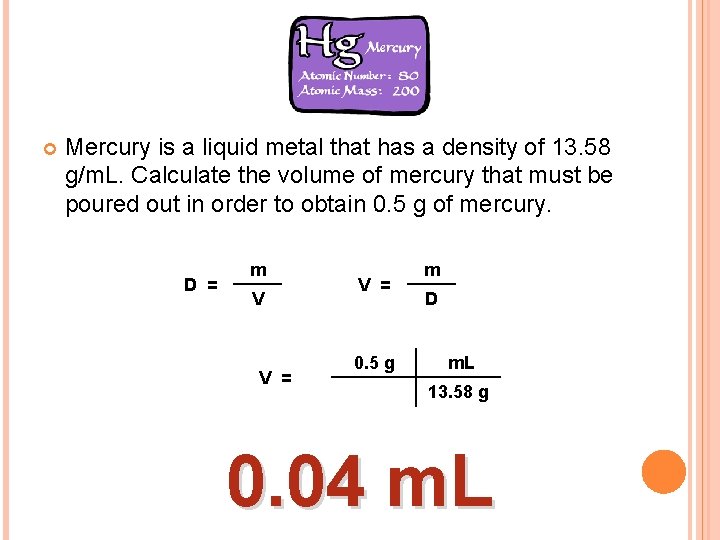
Mercury is a liquid metal that has a density of 13. 58 g/m. L. Calculate the volume of mercury that must be poured out in order to obtain 0. 5 g of mercury. D = m V V = 0. 5 g m D m. L 13. 58 g 0. 04 m. L

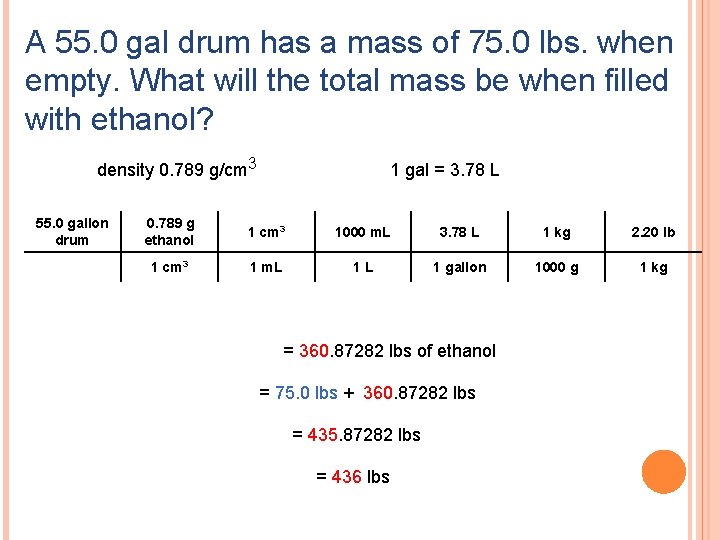
A 55. 0 gal drum has a mass of 75. 0 lbs. when empty. What will the total mass be when filled with ethanol? density 0. 789 g/cm 3 55. 0 gallon drum 1 gal = 3. 78 L 0. 789 g ethanol 1 cm 3 1000 m. L 3. 78 L 1 kg 2. 20 lb 1 cm 3 1 m. L 1 L 1 gallon 1000 g 1 kg = 360. 87282 lbs of ethanol = 75. 0 lbs + 360. 87282 lbs = 435. 87282 lbs = 436 lbs

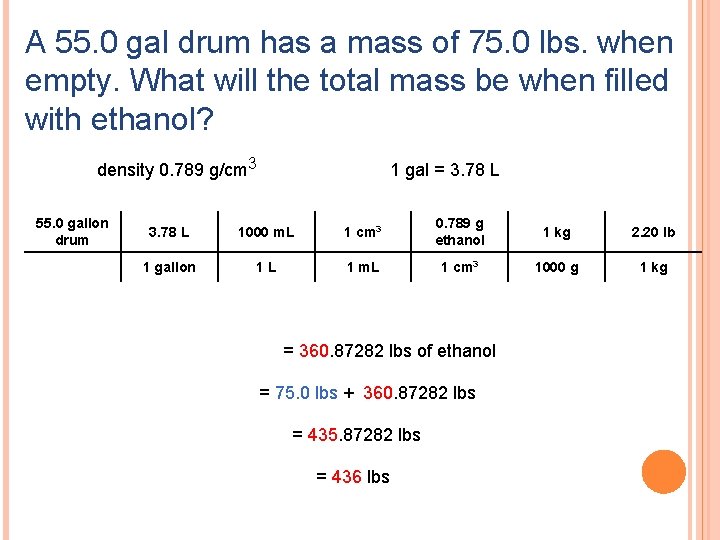
A 55. 0 gal drum has a mass of 75. 0 lbs. when empty. What will the total mass be when filled with ethanol? density 0. 789 g/cm 3 55. 0 gallon drum 1 gal = 3. 78 L 1000 m. L 1 cm 3 0. 789 g ethanol 1 kg 2. 20 lb 1 gallon 1 L 1 m. L 1 cm 3 1000 g 1 kg = 360. 87282 lbs of ethanol = 75. 0 lbs + 360. 87282 lbs = 435. 87282 lbs = 436 lbs

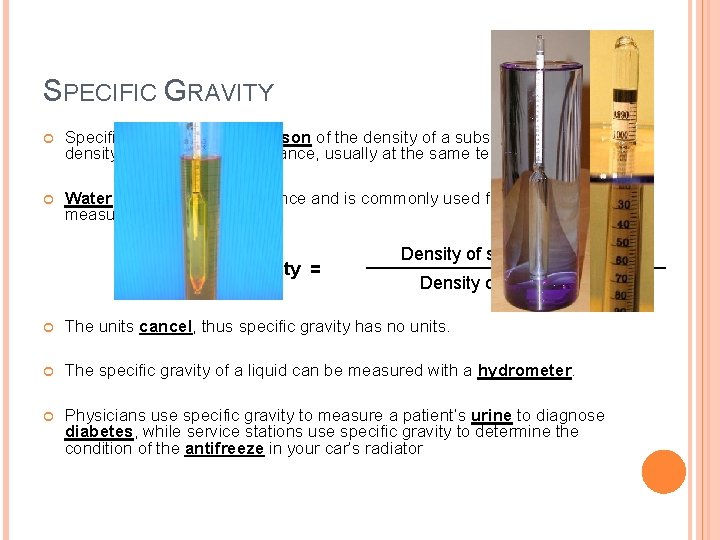
SPECIFIC GRAVITY Specific gravity is a comparison of the density of a substance to the density of a reference substance, usually at the same temperature. Water is a convenient reference and is commonly used for this measurement. Specific Gravity = Density of substance (g/cm 3) Density of water (g/cm 3) The units cancel, thus specific gravity has no units. The specific gravity of a liquid can be measured with a hydrometer. Physicians use specific gravity to measure a patient’s urine to diagnose diabetes, while service stations use specific gravity to determine the condition of the antifreeze in your car’s radiator