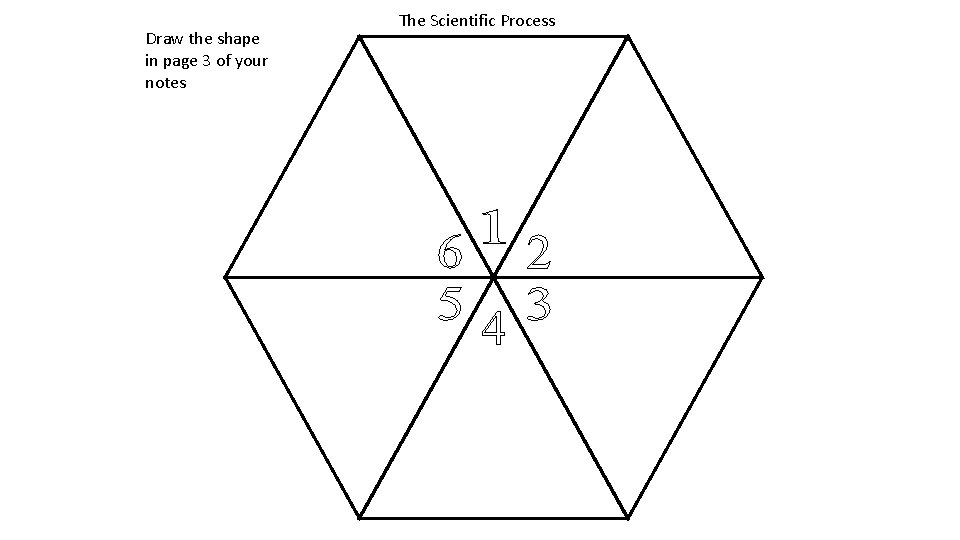
Scientific Method Draw the shape in page 3









![Base Units • meters (m) – length [Area - m 2] • (km) – Base Units • meters (m) – length [Area - m 2] • (km) –](https://slidetodoc.com/presentation_image_h/f7baa922f4d6baa210109a5894626b72/image-22.jpg)













![Hypothesis • If ___[I do this]___, then ___[this will happen]___. IV DV • Question: Hypothesis • If ___[I do this]___, then ___[this will happen]___. IV DV • Question:](https://slidetodoc.com/presentation_image_h/f7baa922f4d6baa210109a5894626b72/image-50.jpg)


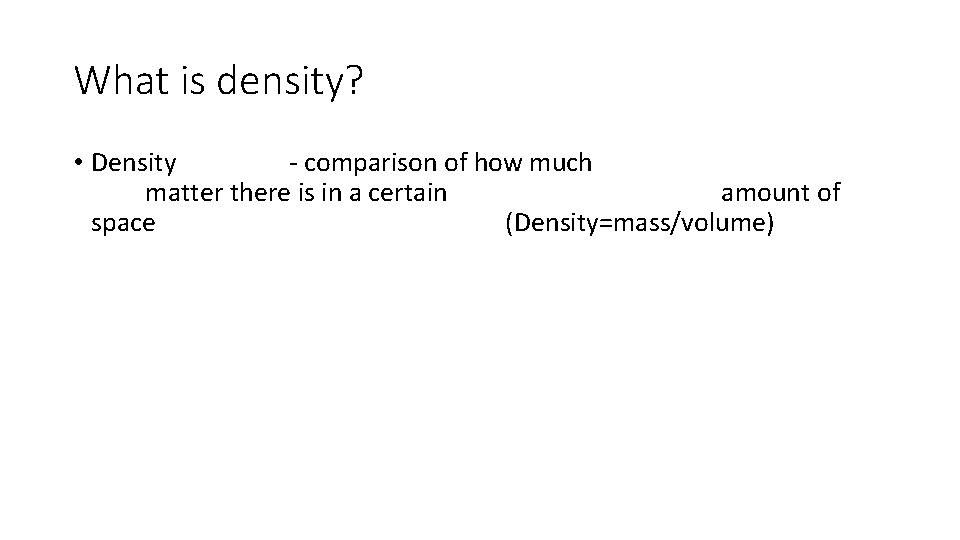












- Slides: 71

Scientific Method

Draw the shape in page 3 of your notes The Scientific Process








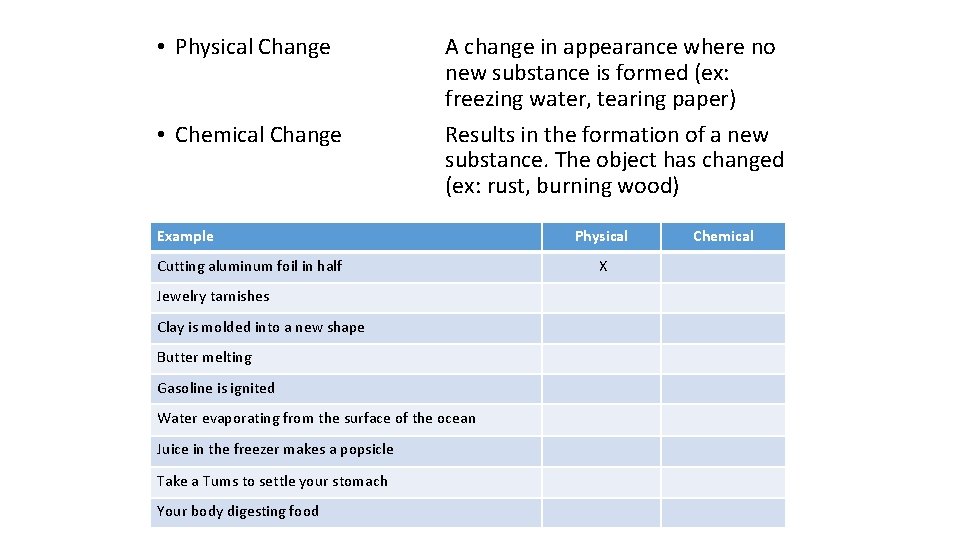
• Physical Change • Chemical Change A change in appearance where no new substance is formed (ex: freezing water, tearing paper) Results in the formation of a new substance. The object has changed (ex: rust, burning wood) Example Cutting aluminum foil in half Jewelry tarnishes Clay is molded into a new shape Butter melting Gasoline is ignited Water evaporating from the surface of the ocean Juice in the freezer makes a popsicle Take a Tums to settle your stomach Your body digesting food Physical X Chemical

• Observation any information collected with the senses • Inference Logical explanation of an observation that is drawn from prior knowledge or experience

Observation In the space below, record 5 observations about your science classroom. 1. 2. 3. 4. 5.

List 3 inferences about the classroom 1. 2. 3.

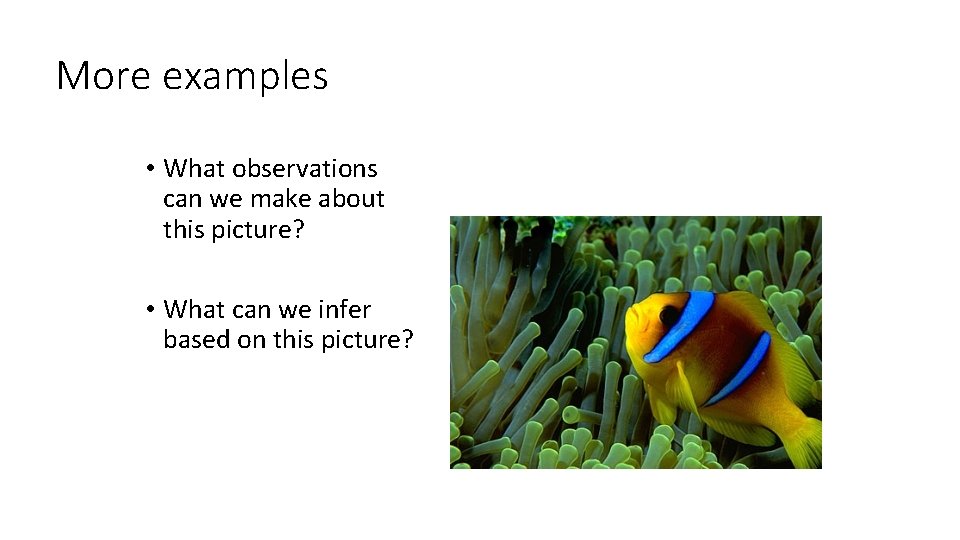
More examples • What observations can we make about this picture? • What can we infer based on this picture?

What observations can we make from these pictures?

• Qualitative • Quantitative Descriptions, can be observed but not measured (ex: Colors, textures, smells, tastes, appearance, beauty, etc. ) Deals with numbers, data that can be measured (ex: Length, height, area, volume, weight, speed, time, temperature, ages, etc. )

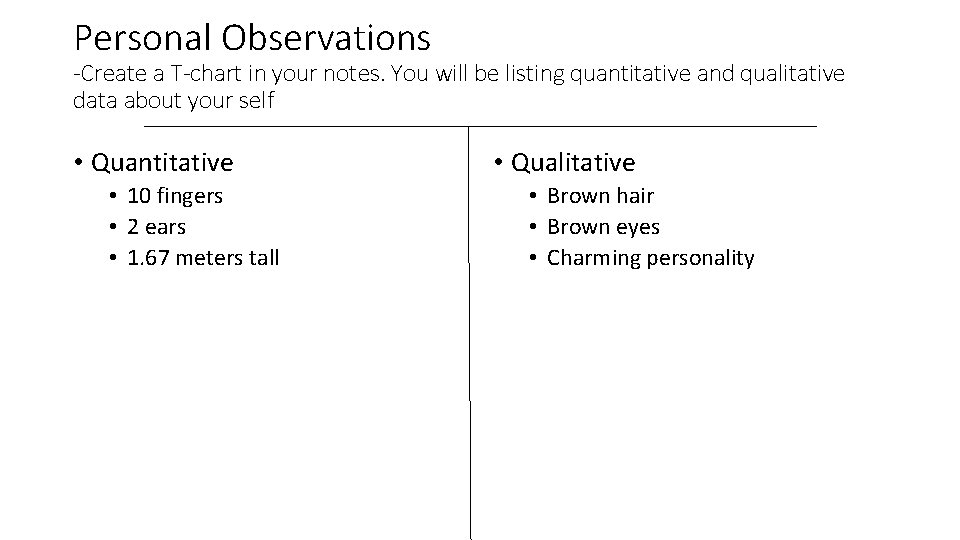
Personal Observations -Create a T-chart in your notes. You will be listing quantitative and qualitative data about your self • Quantitative • 10 fingers • 2 ears • 1. 67 meters tall • Qualitative • Brown hair • Brown eyes • Charming personality

Answer the questions. • Which of the pieces of the paper do you think experienced chemical changes? Explain your answers. • Which of the pieces of paper do you think experienced physical changes? Explain your answer. • In your own words, write a sentence that differentiates chemical from physical change. • If you were asked to prepare an envelope of items for another student so they could identify physical and chemical changes, what items would you select and how could you modify each one to show they of change?



Estimate Lab • In this lab you will be asked to estimate the size (mass, volume, length) of various objects. All measurements will be recorded using metric units.
![Base Units meters m length Area m 2 km Base Units • meters (m) – length [Area - m 2] • (km) –](https://slidetodoc.com/presentation_image_h/f7baa922f4d6baa210109a5894626b72/image-22.jpg)
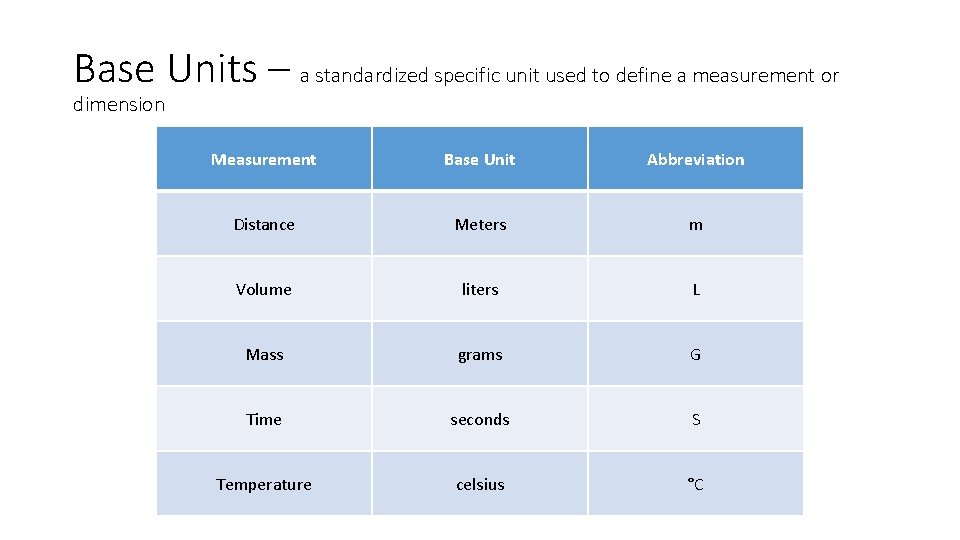
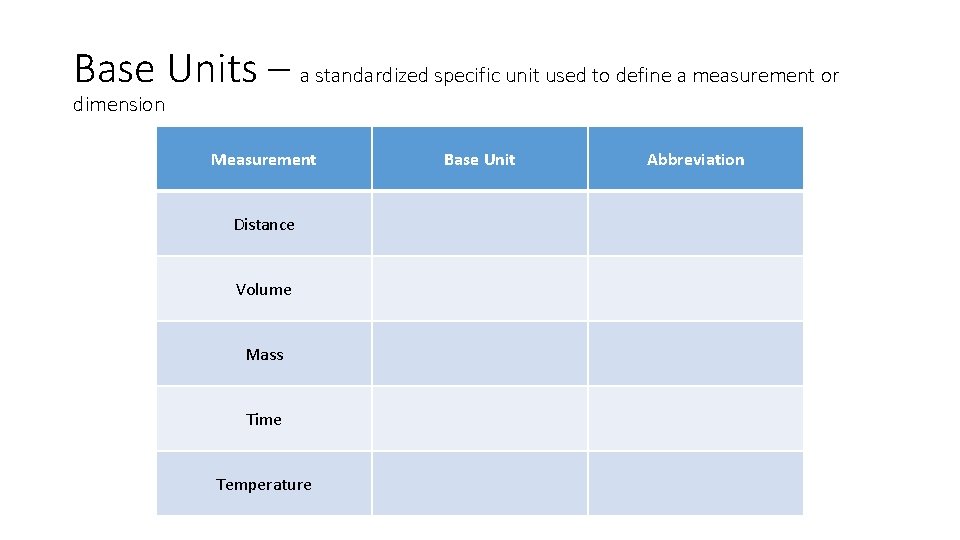
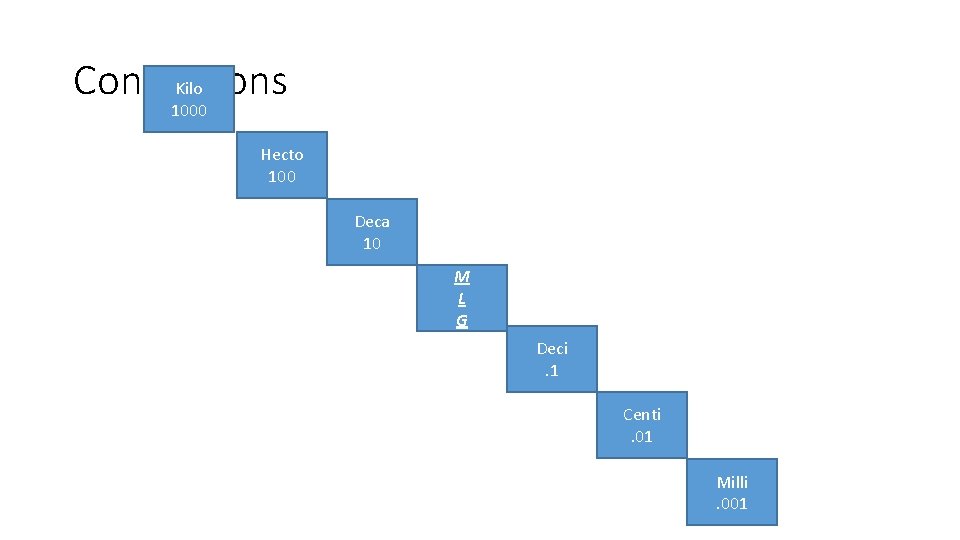
Base Units • meters (m) – length [Area - m 2] • (km) – long distances, (m) white boards, (cm) pencils, posters, (mm) lead, hair • Liters (L) – volume – how much space a solid, liquid, or gas takes up • (k. L) swimming pools, (L) two liter soda bottles, (m. L) personal water bottles • grams (g) – mass – how much matter makes up an object (NOT weight, weight is based on gravity) • (kg) TV, (g) pencil, binder (mg) sheet of paper • °Celsius – (°C) Kilo Hecto Deka *meters *Liters *Grams Base Units Deci Centi Milli

Base Units – a standardized specific unit used to define a measurement or dimension Measurement Base Unit Abbreviation Distance Meters m Volume liters L Mass grams G Time seconds S Temperature celsius °C

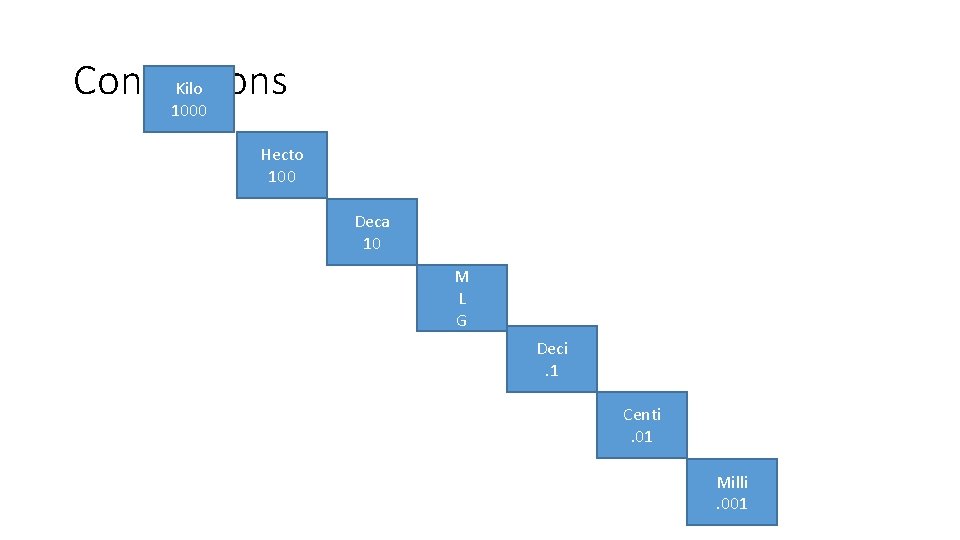
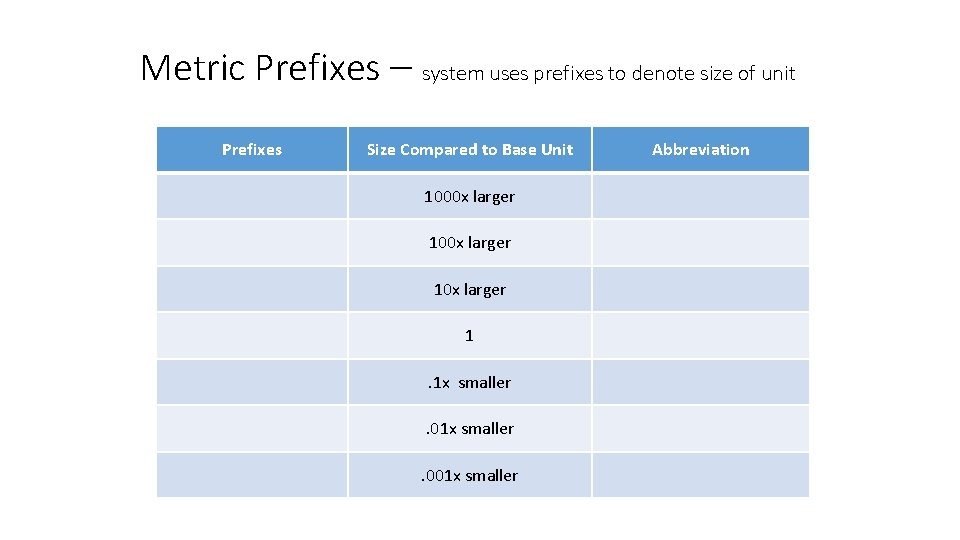
Metric Prefixes – system uses prefixes to denote size of unit Prefixes Size Compared to Base Unit Abbreviation Kilo 1000 x larger k Hecto 100 x larger h Deca 10 x larger da Base Unit 1 Whatever the base is Deci . 1 x smaller d Centi . 01 x smaller c Milli . 001 x smaller m

Conversions Kilo 1000 Hecto 100 Deca 10 M L G Deci. 1 Centi. 01 Milli. 001

Base Units – a standardized specific unit used to define a measurement or dimension Measurement Distance Volume Mass Time Temperature Base Unit Abbreviation

Metric Prefixes – system uses prefixes to denote size of unit Prefixes Size Compared to Base Unit 1000 x larger 10 x larger 1. 1 x smaller. 001 x smaller Abbreviation

Conversions Kilo 1000 Hecto 100 Deca 10 M L G Deci. 1 Centi. 01 Milli. 001

• Kangaroos • Hop • Down • Under • Drinking • Chocolate • Milk • Kilo • Hecta • Deka- (Deca-) • Unit (gram, meter, liter) • Deci • Centi • Milli-

Metric Conversions 1. Write out KHD(m, L, g)DCM 2. Circle the appropriate unit 3. Move the appropriate number of decimal points towards the ending units. 4. Check your work! 50 km = ______ m 3. 9 g = ______ kg 28 m. L = ______ c. L 15 kg = ______ cg



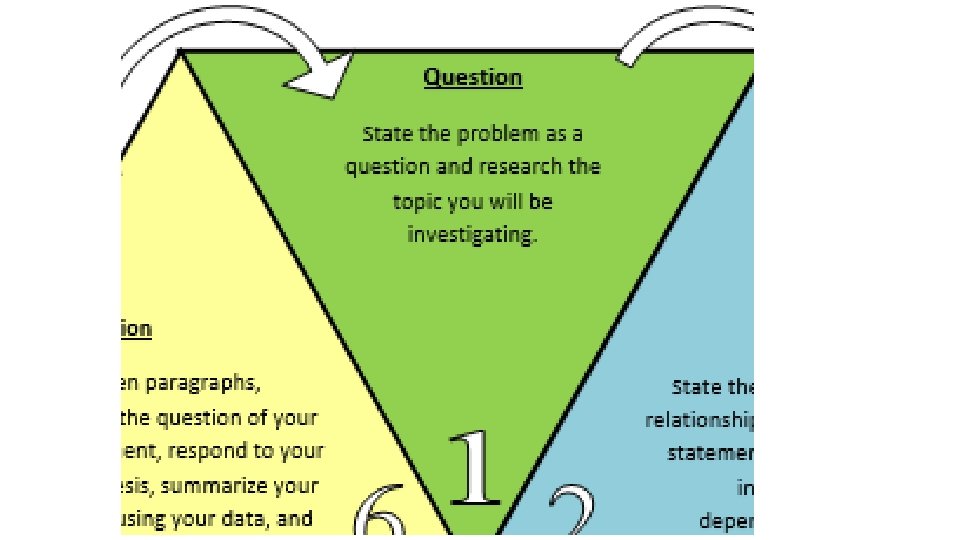
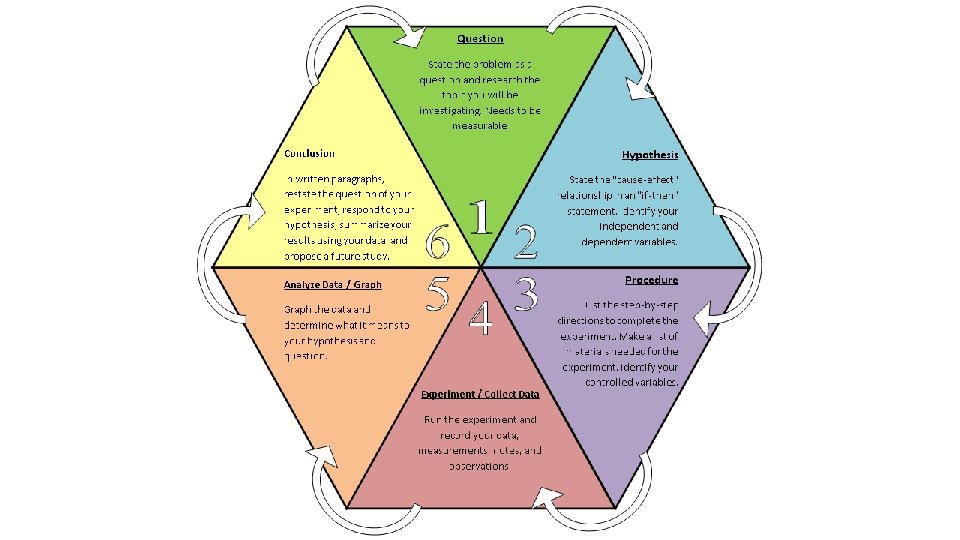
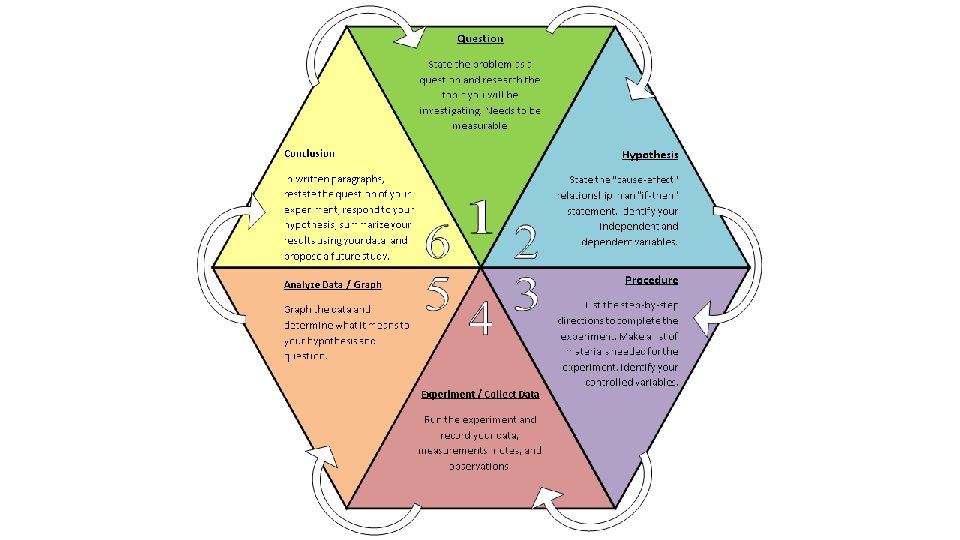
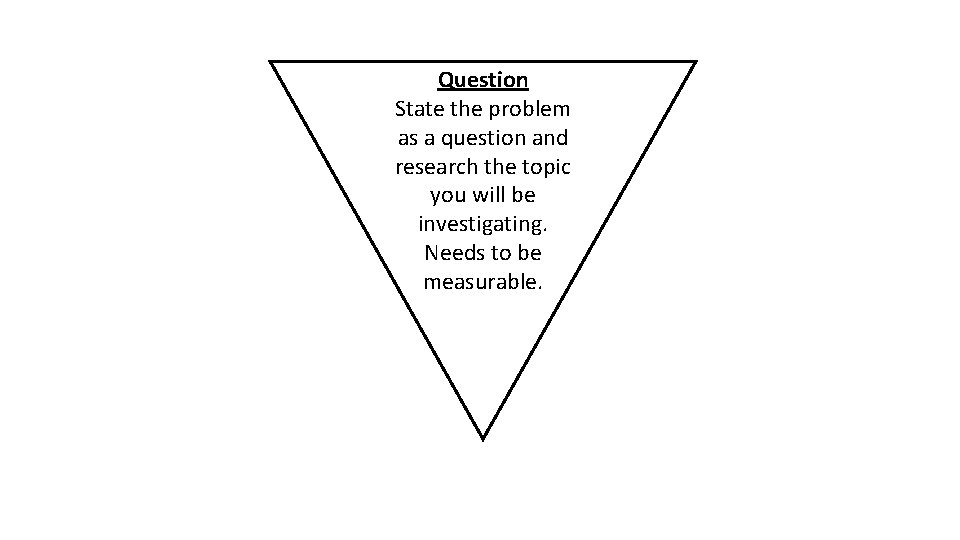
Question State the problem as a question and research the topic you will be investigating. Needs to be measurable.

Questioning characteristics • Ask questions about objects, organisms, and events in the natural world. • Can be answered through investigations that involve experiments, observations or surveys. • Answered by collecting and analyzing evidence that is measurable • Relate to scientific ideas rather than personal preference or moral values • Do not related to the supernatural or non-measurable phenomena

Questioning - Shows cause and effect relationship and must be measurable. • What type of soil is best to grow tomato plants? • Cause and Effect Relationship • Cause: types of soil • Effect: growth of plant • Measurable • Measurement: growth/size of tomato plant

Rephrase the general question to make it testable 1. 2. 3. 4. 5. 6. 7. How is bug blood different from human blood? Why do your fingers wrinkle after taking a bath? Is rock music better than hip hop music? Why does bright light cause some people to sneeze? Does smell affect people's moods? Is vegetarianism better than eating meat? Which battery is best?


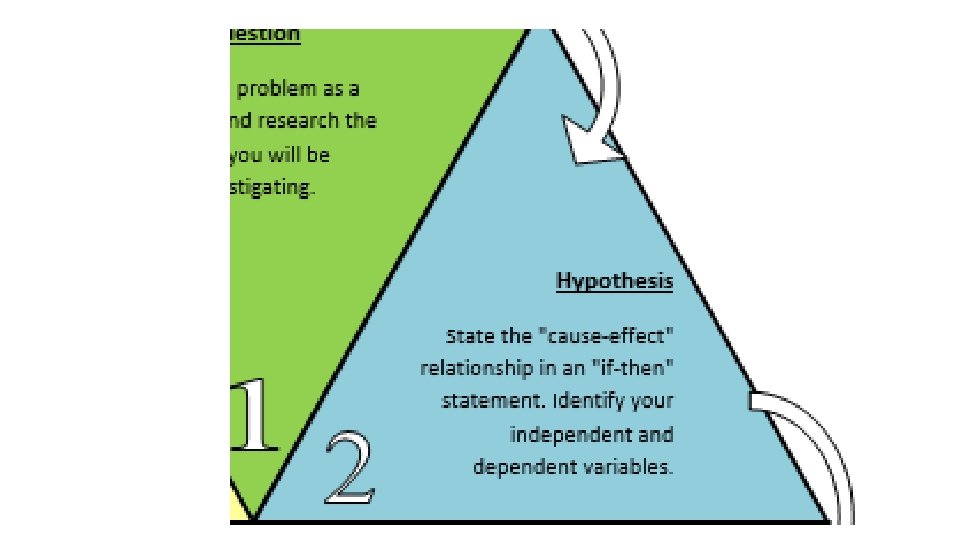
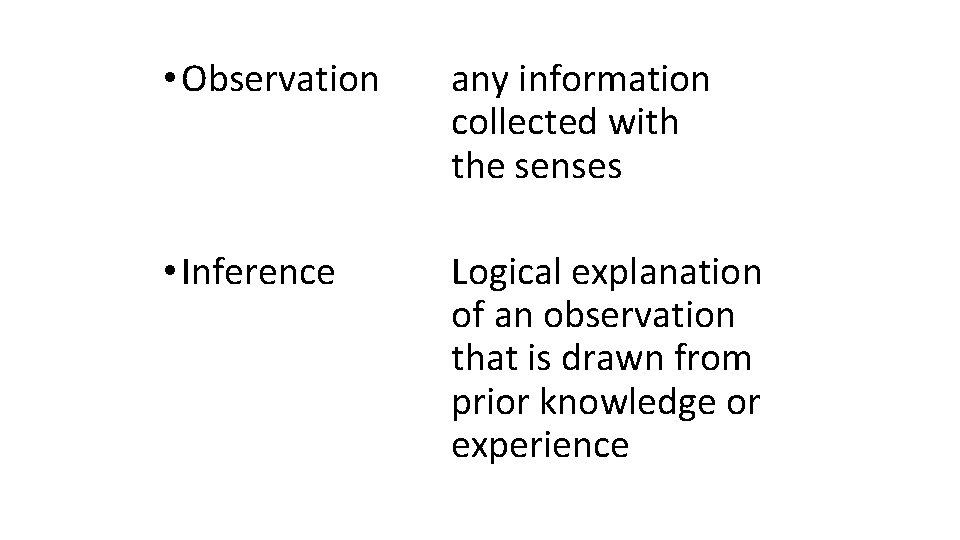
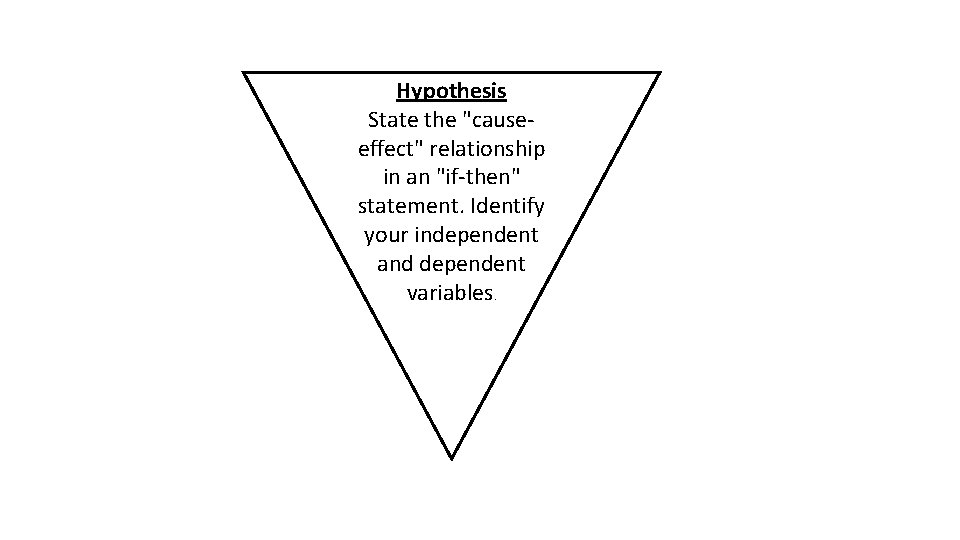
Hypothesis State the "causeeffect" relationship in an "if-then" statement. Identify your independent and dependent variables.

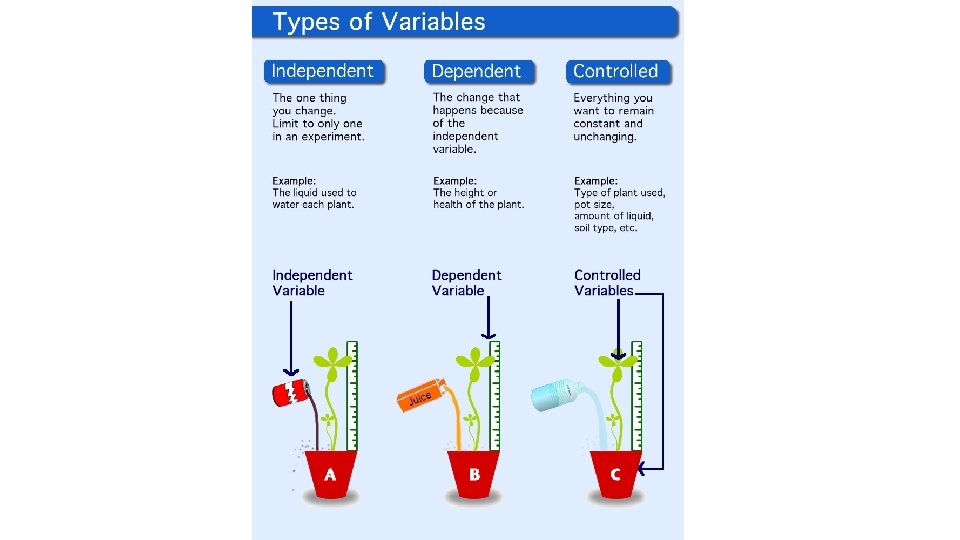
• Variable any factor in an experiment that can have more than one value. • Dependent variable factor that is measured or (DV) observed during an experiment • Independent variable factor that you want to test; (IV) it is changed by the investigator to observe how it affects a dependent variable; also known as a manipulated variable • Controls (constants) the factors in the experiment (CV) that remain the same


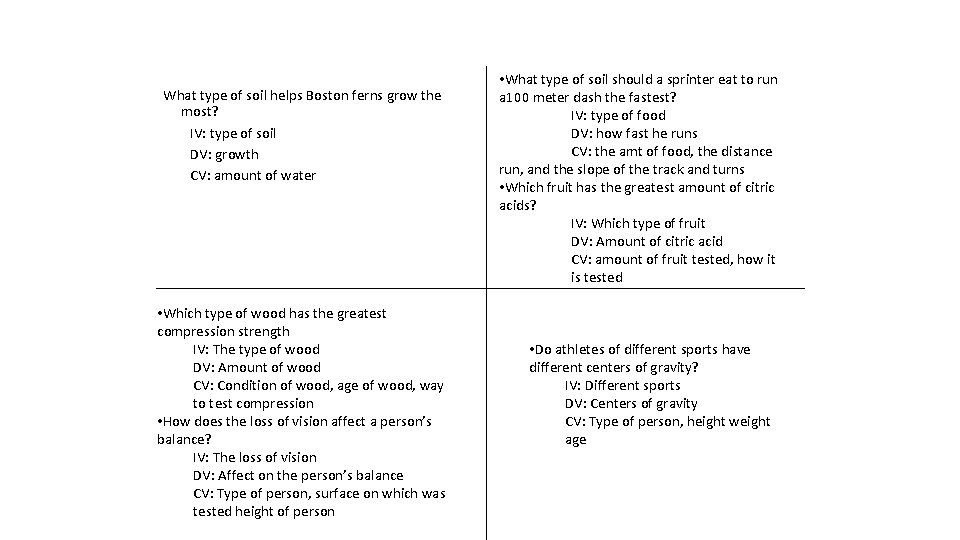
Q: What type of soil helps Boston ferns grow the most? IV: DV: CV: Q: Which type of wood has the greatest compression strength? IV: DV: CV: Q: How does the loss of vision affect a person’s balance? IV: DV: CV: Q: What type of food should a sprinter eat to run a 100 -meter dash the fastest? IV: DV: CV: Q: Which fruit has the greatest amount of citric acids? IV: DV: CV: Q: Do athletes of different sports have different centers of gravity? IV: DV: CV:

What type of soil helps Boston ferns grow the most? IV: type of soil DV: growth CV: amount of water • Which type of wood has the greatest compression strength IV: The type of wood DV: Amount of wood CV: Condition of wood, age of wood, way to test compression • How does the loss of vision affect a person’s balance? IV: The loss of vision DV: Affect on the person’s balance CV: Type of person, surface on which was tested height of person • What type of soil should a sprinter eat to run a 100 meter dash the fastest? IV: type of food DV: how fast he runs CV: the amt of food, the distance run, and the slope of the track and turns • Which fruit has the greatest amount of citric acids? IV: Which type of fruit DV: Amount of citric acid CV: amount of fruit tested, how it is tested • Do athletes of different sports have different centers of gravity? IV: Different sports DV: Centers of gravity CV: Type of person, height weight age

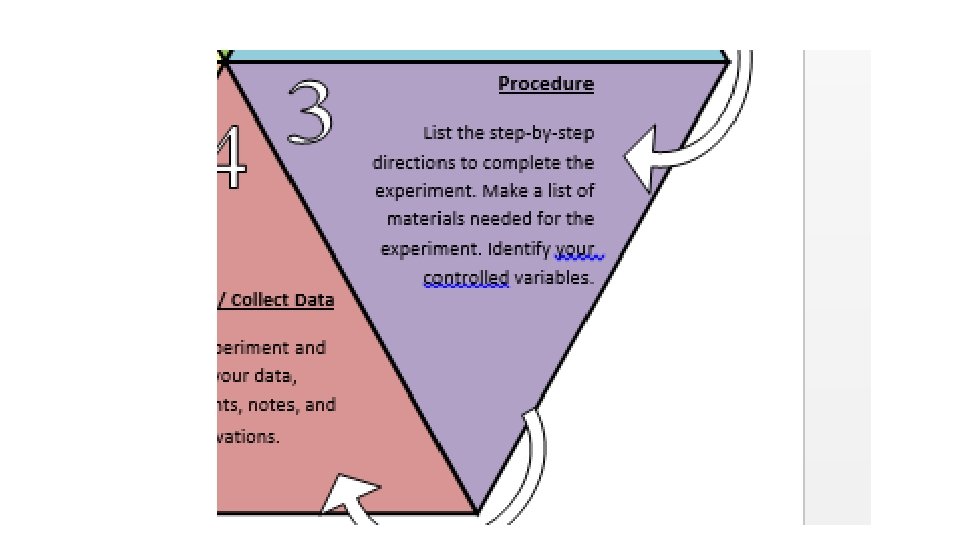
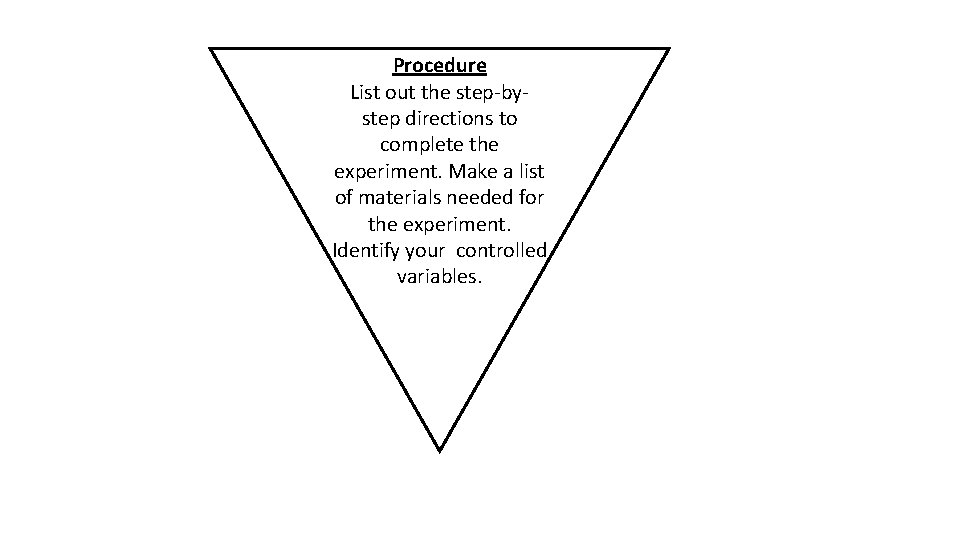
Procedure List out the step-bystep directions to complete the experiment. Make a list of materials needed for the experiment. Identify your controlled variables.

Build a Box • You will be given 3 sheets of construction paper, a foot of tape and a pair of scissors • Your job will be to build a box out of construction paper however big you choose. • You don’t have to use all of the paper • You can cut the paper however you want • As you build, write the step by step procedure on how to construct it, so that someone else will be able to build it the exact same way that you did. • Don’t forget to put your name on your box!!!


Metric Practice Schmidt-tastic Parcel Company specializes in shipping parcels. The cost of shipping a package is $1. 76 for each half of a kilogram shipped. Frida (8 kg 22 g), Samuel (404 g), Brittany (9 kg), Brandon(903 g), and Aaron (12. 9 kg) each have one package to ship. 1. Which package has the most mass? 2. Whose package costs more to ship? How much does it cost?

Metric check 1. 1 cm = _____mm 2. 1000 g = _____kg 3. 5 k. L = ____L 4. 12. 4 km = _____m 5. 1. 2 kg = ____g 6. 3000 g = _______kg 7. 2 L = _____m. L 8. 3456 mm = ____m 9. 1 km = _______m 10. 1400 mg = _____g 1. 10 mm 2. 1 kg 3. 5000 L 4. 12400 m 5. 1200 g 6. 3 kg 7. 2000 m. L 8. 3. 456 m 9. 1000 m 10. 1. 4 g

Homer notices that his shower is covered in a strange green slime. His friend Barney tells him that coconut juice will get rid of the green slime. Homer decides to check this out by spraying half of the shower with coconut juice. He sprays the other half of the shower with water. After 3 days of "treatment" there is no change in the appearance of the green slime on either side of the shower. Identify the 1. What was the initial observation? 2. Control Group 3. Independent Variable 4. Dependent Variable 5. What should Homer's conclusion be?

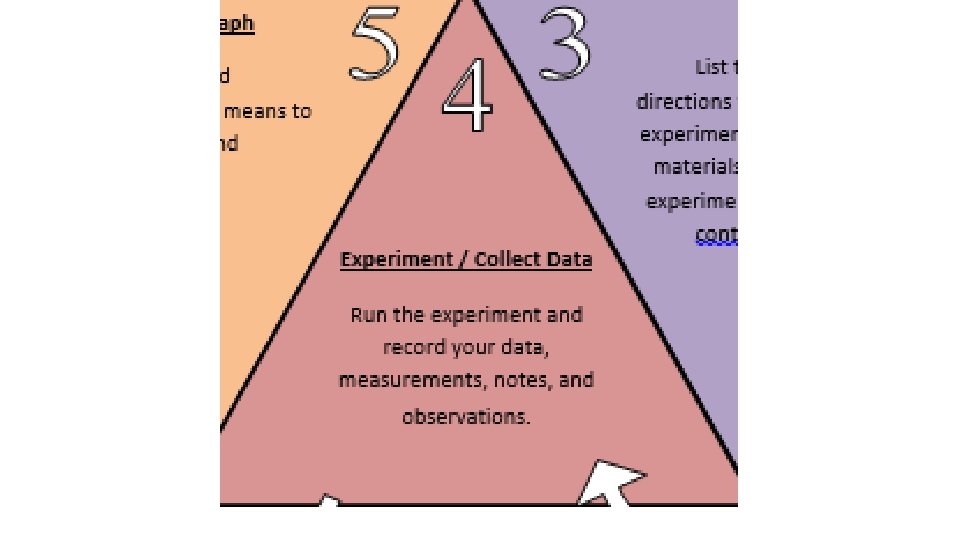
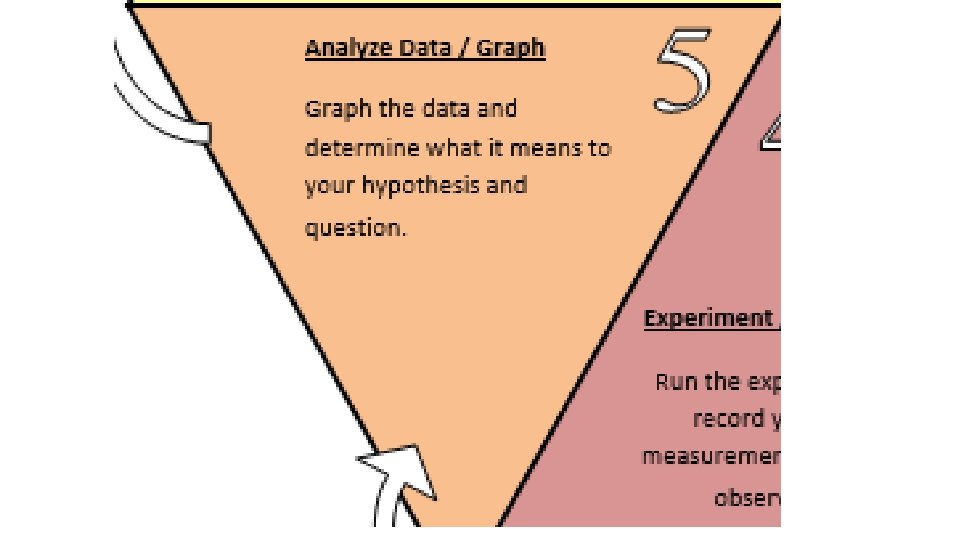
Analyze Data/Graph the data and determine what it means to your hypothesis and question
![Hypothesis If I do this then this will happen IV DV Question Hypothesis • If ___[I do this]___, then ___[this will happen]___. IV DV • Question:](https://slidetodoc.com/presentation_image_h/f7baa922f4d6baa210109a5894626b72/image-50.jpg)
Hypothesis • If ___[I do this]___, then ___[this will happen]___. IV DV • Question: Which seeds germinate quicker, carrot seeds or tomato seeds? Hypothesis: If I plant carrot seeds and tomato seeds, then carrot seeds will germinate quicker. • Question: Which bread molds quickest: sourdough bread, whole wheat bread, or enriched white bread. Hypothesis: If I place sourdough bread, whole wheat bread, and enriched white bread in a moist, plastic bag, then whole wheat bread will mold the quickest. • Question: Which type of dog food does Pebbles like best: dry food or moist food? Hypothesis: If I give Pebbles a choice of dry food or moist food, then he will eat the moist food first.

Question to Hypothesis Practice • Try turning these three questions into hypothesis using “If…. then…. ” statements. 1. What effect does temperature have on the elasticity of a rubber band? IV: DV: CV: Hypothesis: 2. Which releases more heat when burned, a peanut or a pecan? IV: DV: CV: Hypothesis 3. How does temperature affect the rate of seed germination in pumpkin seeds? IV: DV: CV: Hypothesis

There are four boxes (a pink box, a purple box, a white box, and a yellow box). Each box has a different length (57 cm, 42 cm, 23 cm 4 mm, and 34 cm 5 mm), a different width (4 cm 2 mm, 8 cm 4 mm, 4 cm 7 mm, and 6 cm 3 mm), and a different height (65 cm 9 mm, 94 cm 7 mm, 55 cm 1 mm, and 43 cm 9 mm). Figure out the length, width, height, and volume for each box. 1. One box has a length of 42 cm and a height of 55 cm 1 mm. 2. One box has a width of 8 cm 4 mm and a height of 94 cm 7 mm. 3. The length of the white box is 0. 234 meters. 4. The yellow box has the smallest width. 5. The white box has the largest height. 6. If the length of purple box was increased by 2 cm, the volume of purple box would increase by 553, 140 cubic millimeters. 7. The volume of the yellow box is 9, 548, 910 cubic millimeters.
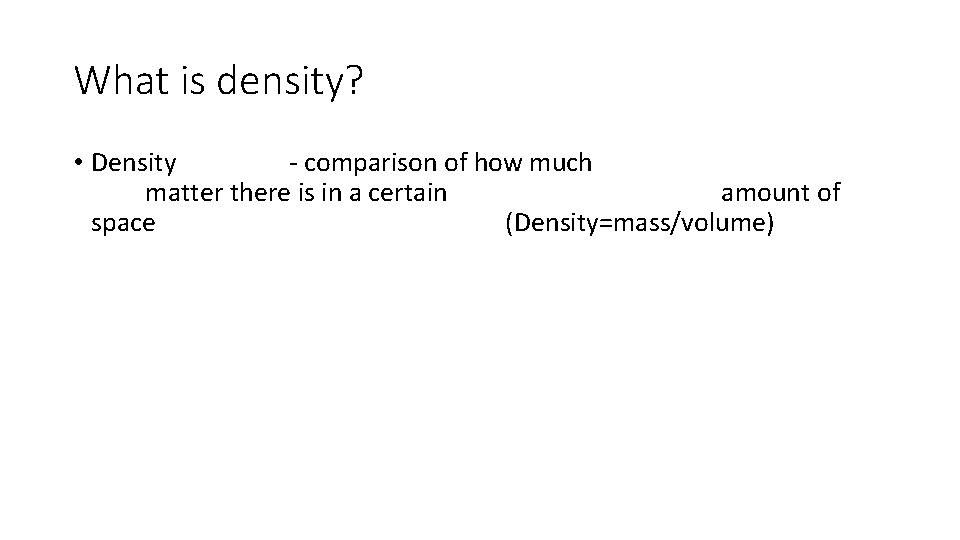
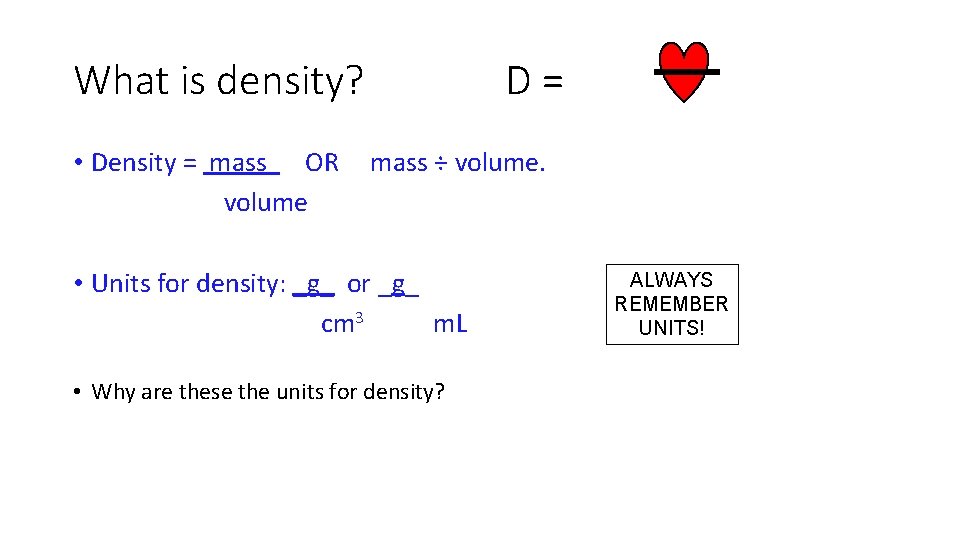
What is density? • Density - comparison of how much matter there is in a certain amount of space (Density=mass/volume)

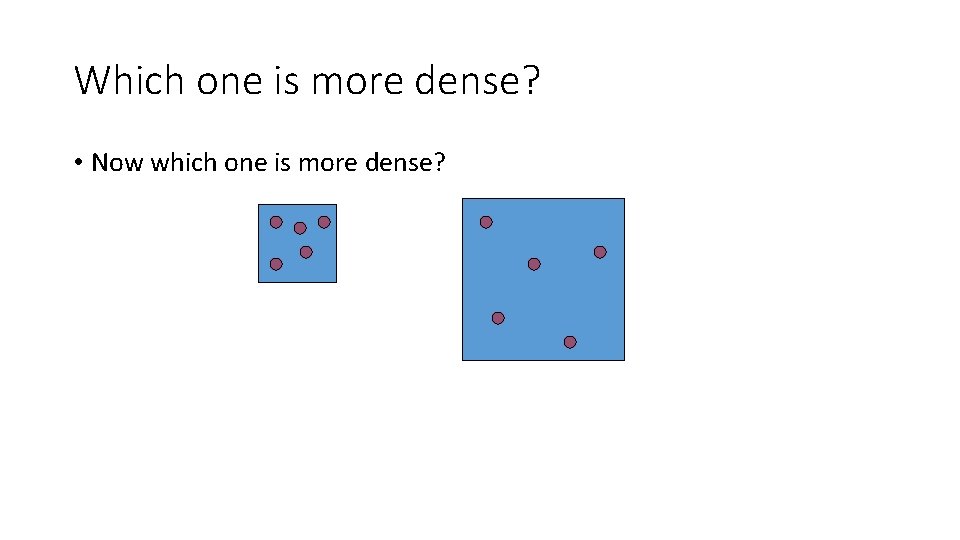
Which one is more dense? • Now which one is more dense?

What is density? D= • Density = mass OR mass ÷ volume • Units for density: _g_ or _g_ cm 3 m. L • Why are these the units for density? ALWAYS REMEMBER UNITS!

Density • Frank has a paper clip. It has a mass of 9 g and a volume of 3 cm 3. What is its density? • Frank also has an eraser. It has a mass of 3 g, and a volume of 1 cm 3. What is its density? • Jack has a rock. The rock has a mass of 6 g and a volume of 3 cm 3. What is the density of the rock? • Jill has a gel pen. The gel pen has a mass of 8 g and a volume of 2 cm 3. What is the density of the rock?

Density Problems 1. What is the density of a piece of wood that has a mass of 25. 0 grams and a volume of 29. 4 cm 3? 2. A piece of wood that measures 3. 0 cm by 6. 0 cm by 4. 0 cm has a mass of 80. 0 grams. What is the density of the wood? Would the piece of wood float in water? (volume = L x W x H) 3. A cup of gold colored metal beads was measured to have a mass 425 grams. By water displacement, the volume of the beads was calculated to be 48. 0 cm 3. Given the following densities, identify the metal. • Gold: 19. 3 g/m. L • Copper: 8. 86 g/m. L • Bronze: 9. 87 g/m. L

Answers 1. 0. 850 g/cm 3 2. 1. 1 g/cm 3; No, it would not float on water 3. Copper, 8. 86 g/m. L

Experiment /Collect Data Run the experiment and record your data, measurements, notes, and observations.

TAILS-G D R Y Title – appropriate and descriptive; not cutesy or catchy Axes – appropriate variable on each; labeled “x” and “y” Interval – evenly spread throughout Labels – both axes, general and specific; include measurement Scale – appropriate for the data Graph – Graph the data dependent responding y-axis MIX Manipulated Independent X-axis

Analyze Data / Graph the data and determine what it means to your hypothesis and question.

Create Your Own Experiment • You will be divided into groups of 3 -4 • You will be given a bag with materials in it • Your job will be to create an experiment using those materials. • Remember that you can only have one IV • You will be collecting data, graphing and analyzing it, so as you are coming up with the experiment, keep that in mind.

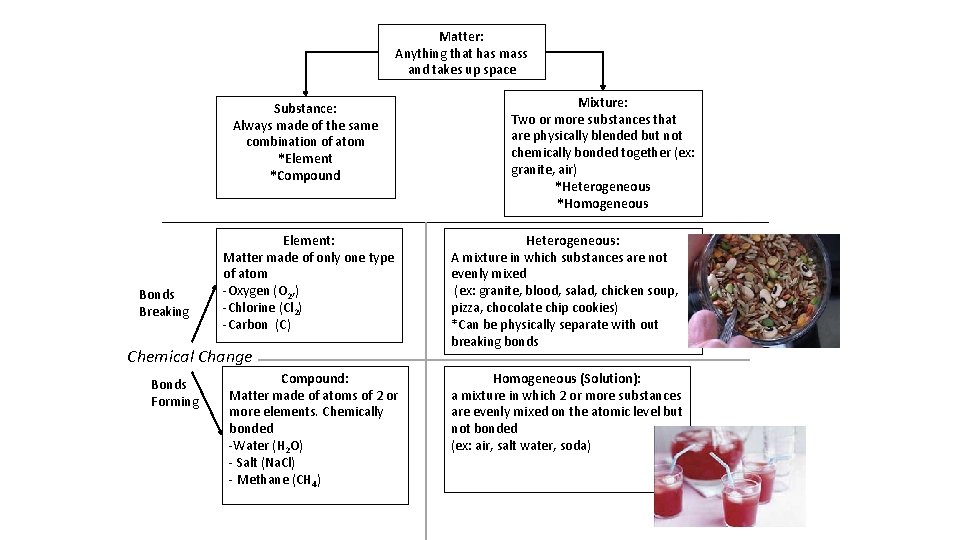
Matter: Anything that has mass and takes up space Substance: Always made of the same combination of atom *Element *Compound Bonds Breaking Element: Matter made of only one type of atom -Oxygen (O 2, ) -Chlorine (Cl 2) -Carbon (C) Chemical Change Bonds Forming Compound: Matter made of atoms of 2 or more elements. Chemically bonded -Water (H 2 O) - Salt (Na. Cl) - Methane (CH 4) Mixture: Two or more substances that are physically blended but not chemically bonded together (ex: granite, air) *Heterogeneous *Homogeneous Heterogeneous: A mixture in which substances are not evenly mixed (ex: granite, blood, salad, chicken soup, pizza, chocolate chip cookies) *Can be physically separate with out breaking bonds Homogeneous (Solution): a mixture in which 2 or more substances are evenly mixed on the atomic level but not bonded (ex: air, salt water, soda)

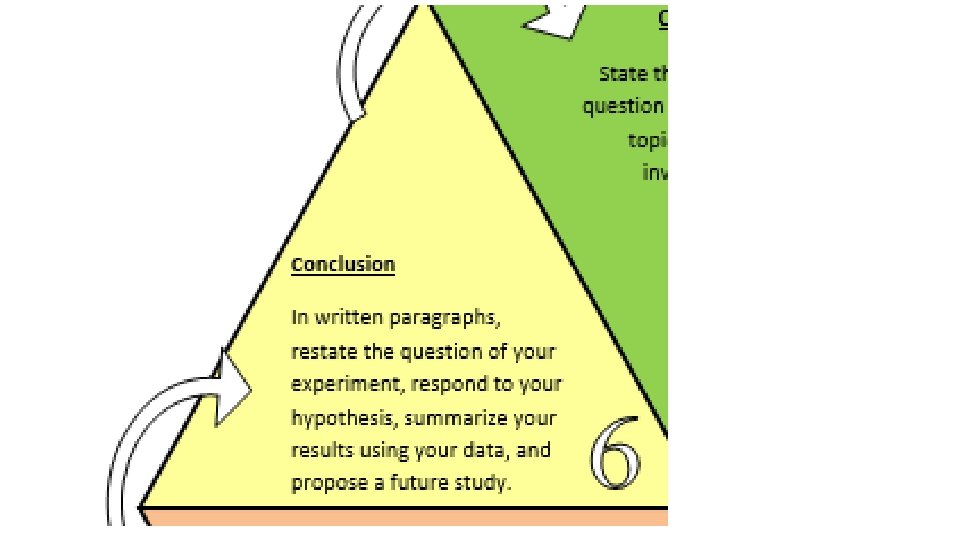
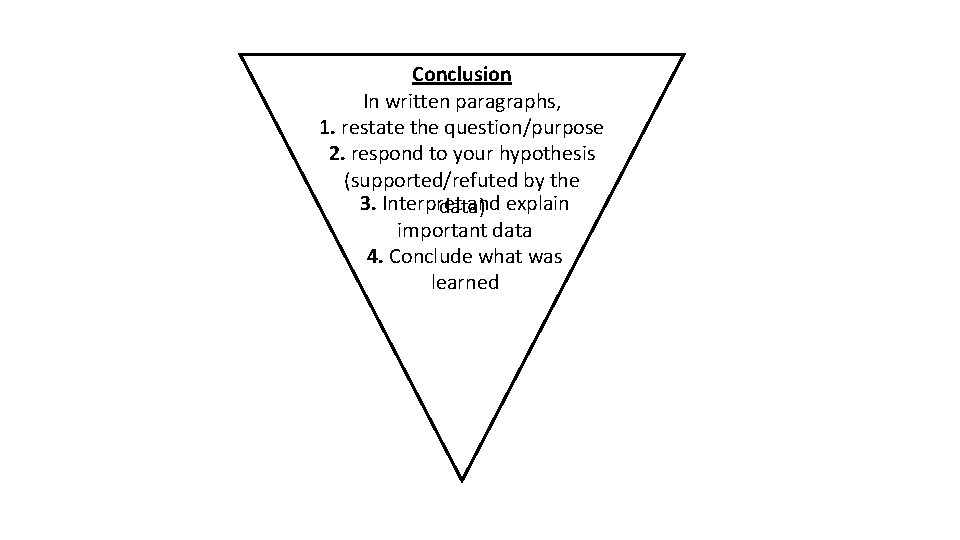
Conclusion In written paragraphs, 1. restate the question/purpose 2. respond to your hypothesis (supported/refuted by the 3. Interpret and explain data) important data 4. Conclude what was learned

7 Point Conclusion • Using the data table & graph, write 1 -paragraph conclusion for this experiment with at least 4 sentences. Include… • restate the problem/question (1 point) • discuss your hypothesis (1 point) • explain the results (don’t forget to include data from the table and graph) (3 points) • suggest a future study that relates to the problem/question (2 points)

Sample 1 We were investigating if there was a relationship between height and arm span. My prediction was supported because there is a relationship between height and arm span. My data supported my prediction because “student A” had a height of 153 and arm span of 147 then “student B” had an arm span of 157 arm span of 148 and “student c” height of 155 arm span of 153. My future study would be to see if height is always taller than arm span.

Sample 2 The length of your arm span and hieght of the 3 students was going to be equal to each other. None of us got an equal measurement to our hieght and arm span. Only one person got their arm span length and hieght closest to be equal that was student A.

Sample 3 In this experiment the question that we were trying to answer the question: Is there a relationship between height and arm span. Our prediction was correct, our prediction was that there is a relationship between arm span and height. Our prediction was correct because we were only 2, 4 inches away from arm span and height. I measured 169 in height and 167 arm span. Student B measured 146 9 cm) in height and 142 9 cm) in arm span. Another experiment that this made me wanna try is: what is the relationship between you hand your head!

Sample 4 Is there a relationship between arm span and height. Are prediction was right except for one person that is arm span and height where different from each other. Are data was correct with the height and arm span. A future study that I would like to do is how the brain works and the different parts

Sample 5 After testing on me and two other people I can concelude that my prediction was correct there is a relationship between height and arm span. Now that I know this I am going to compare the length of my forearm to my foot.

Solute, Solvent, Solution • Mixture of 2 or physically separated • Heterogeneous mixture uniform parts (ex: • Solution the as • Solvent • Solute Matter that can vary in composition, combination more substances that are blended together, can be by physical methods mixture that does not have a blend, can see the different gravel mixture) A mixture in which two or more substances are evenly mixed on atomic level but not bonded together (ex: soda, air); also known a homogenous mixture in a solution the substance that is present in the largest amount all other substances in a solution