Scientific Measurements Scientific Notation In science we deal
































- Slides: 47

Scientific Measurements

Scientific Notation In science, we deal with some very LARGE numbers: 1 mole = 60200000000000 In science, we deal with some very SMALL numbers: Mass of an electron = 0. 000000000000000091 kg

Imagine the difficulty of calculating the mass of 1 mole of electrons! 0. 000000000000000091 kg x 60200000000000 ? ? ? ? ? ? ? ? ?

Scientific Notation: A method of representing very large or very small numbers in the form: M x 10 n Ø M is a number between 1 and 10 Ø n is an integer

. 2 500 000 9 8 7 6 5 4 3 2 1 Step #1: Insert an understood decimal point Step #2: Decide where the decimal must end up so that one number is to its left Step #3: Count how many places you bounce the decimal point Step #4: Re-write in the form M x 10 n

2. 5 x 9 10 The exponent is the number of places we moved the decimal.

0. 0000579 1 2 3 4 5 Step #2: Decide where the decimal must end up so that one number is to its left Step #3: Count how many places you bounce the decimal point Step #4: Re-write in the form M x 10 n

5. 79 x -5 10 The exponent is negative because the number we started with was less than 1.

Write the following in scientific notation: 6 4. 1 x 10 n 4, 100, 000 = ________ 11 3. 456 x 10 n 345, 600, 000 = _____ -2 4. 56 x 10 n 0. 0456= ________ -7 1. 2 x 10 n 0. 00000012=______ -3 3. 05 x 10 n O. 00305= ______

Write the following in standard form: n n n 4. 67 x 103 =_________ 4670 311200 3. 112 x 105 = _________ 0. 0003112 3. 112 x 10 -4 = ________ 4 x 10 -6 = __________ 0. 000004 100, 000, 000 1 x 1011 = _________

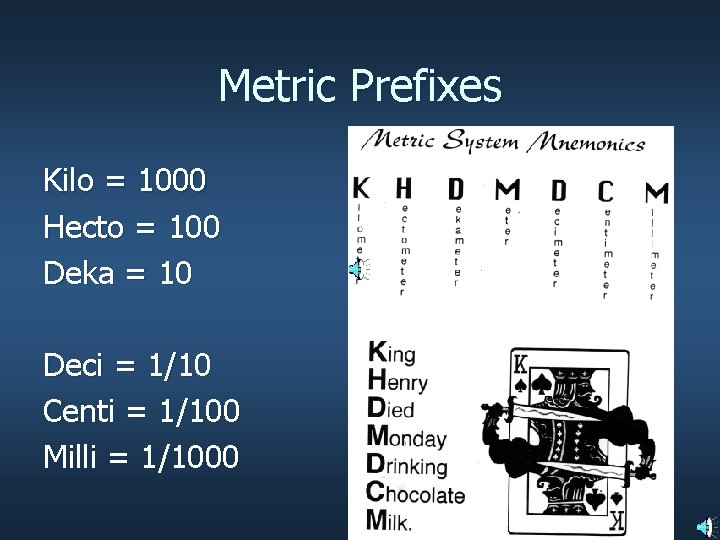
Metric Prefixes Kilo = 1000 Hecto = 100 Deka = 10 Deci = 1/10 Centi = 1/100 Milli = 1/1000

How Big? n n n A meter is about 39 inches A kilometer is 0. 62 mile A centimeter is about the width of your little finger A gram is about the mass of a paper clip A kilogram is 2. 2 pounds 2 liters (pop)

KHDODCM n n n Changing from one metric unit to another is called metric conversion “O” is the space where meter, liter, or gram belongs Kids Have Dropped Over Dead Converting Metrics

KHDODCM n n To change from one metric unit to another, we simply move the decimal point. For example: n n 25. 4 km = _______ cm? K-H-D-O-D-C is 5 places to the right 25. 4 km = 2, 540, 000 cm You always need a number and a unit!

KHDODCM n n n 30 cm = ______hm C – D- O –D- H is 4 places to the left 30 cm = 0. 0030 hm n n (this is the same as 0. 003 hm) 14 dal = _____dl D- O –D is 2 places to the right 14 dal = 1400 dl

SI Units Quantity Base Unit Symbol meter m Mass kilogram kg Time second s Temp kelvin K ampere A Length Current

SI Units Prefix Symbol Factor megakilodecicentimillimicronanopico- M k d c m n p 106 103 10 -1 10 -2 10 -3 10 -6 10 -9 10 -12

Other metric units n n n Temperature is measured in degrees celsius or centigrade Heat is measured in calories or joules Weight is measured in newtons Mega = 1, ooo Micro = one millionth Nano = one billionth

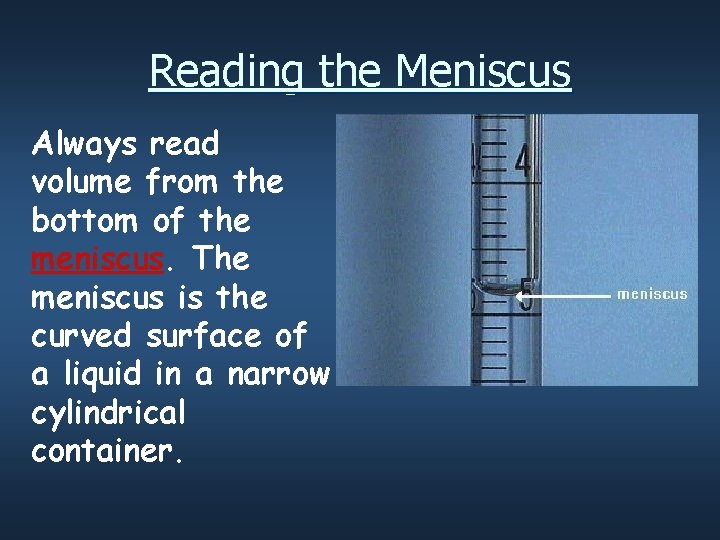
Reading the Meniscus Always read volume from the bottom of the meniscus. The meniscus is the curved surface of a liquid in a narrow cylindrical container.

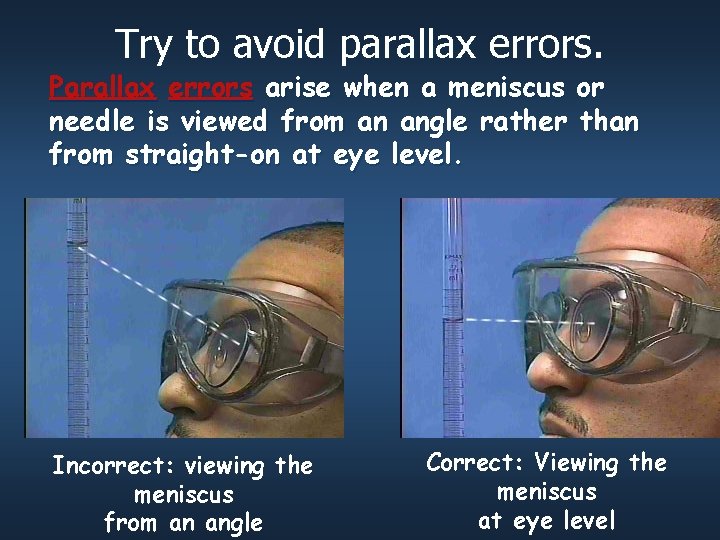
Try to avoid parallax errors. Parallax errors arise when a meniscus or needle is viewed from an angle rather than from straight-on at eye level. Incorrect: viewing the meniscus from an angle Correct: Viewing the meniscus at eye level

Graduated Cylinders The glass cylinder has etched marks to indicate volumes, a pouring lip, and quite often, a plastic bumper to prevent breakage.

Measuring Volume Ø Determine the volume contained in a graduated cylinder by reading the bottom of the meniscus at eye level. Ø Read the volume using all certain digits and one uncertain digit. Ø Certain digits are determined from the calibration marks on the cylinder. ØThe uncertain digit (the last digit of the reading) is estimated.

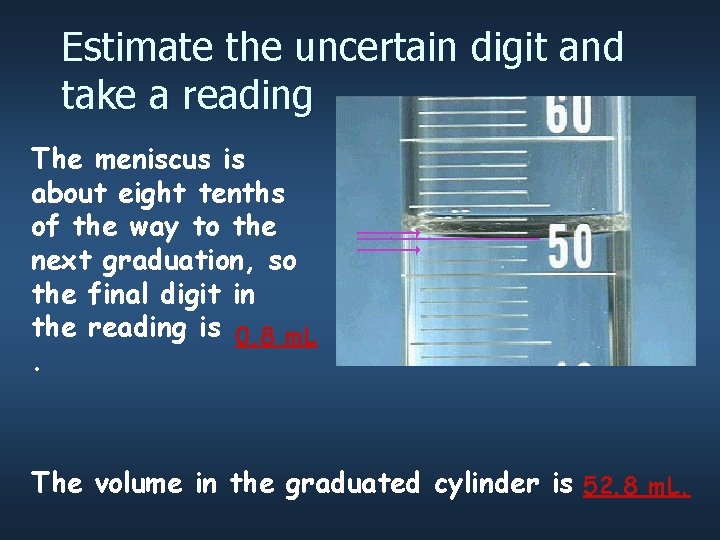
Use the graduations to find all certain digits There are two unlabeled graduations below the meniscus, and each graduation represents 1 m. L, so the certain digits of the reading are… 52 m. L.

Estimate the uncertain digit and take a reading The meniscus is about eight tenths of the way to the next graduation, so the final digit in the reading is 0. 8 m. L. The volume in the graduated cylinder is 52. 8 m. L.

10 m. L Graduate What is the volume of liquid in the graduate? 6 6_ _. _ 2 m. L

25 m. L graduated cylinder What is the volume of liquid in the graduate? 1 5 m. L _1 _. _

100 m. L graduated cylinder What is the volume of liquid in the graduate? 5 7 m. L _2 _. _

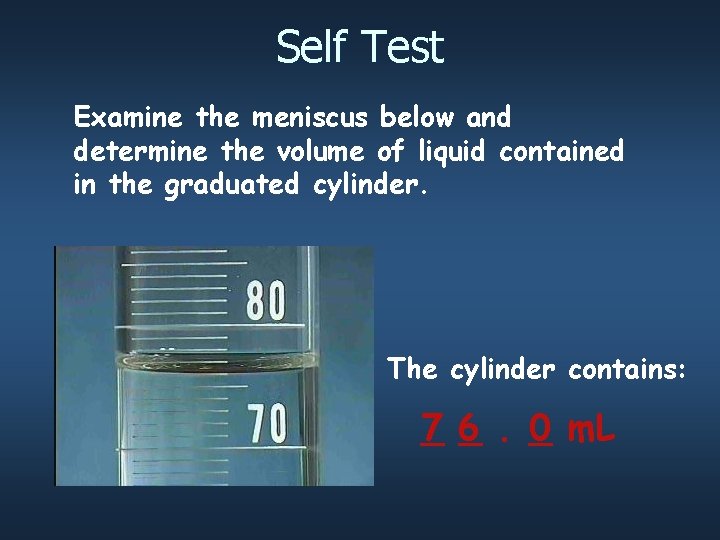
Self Test Examine the meniscus below and determine the volume of liquid contained in the graduated cylinder. The cylinder contains: _6 7 _. _ 0 m. L

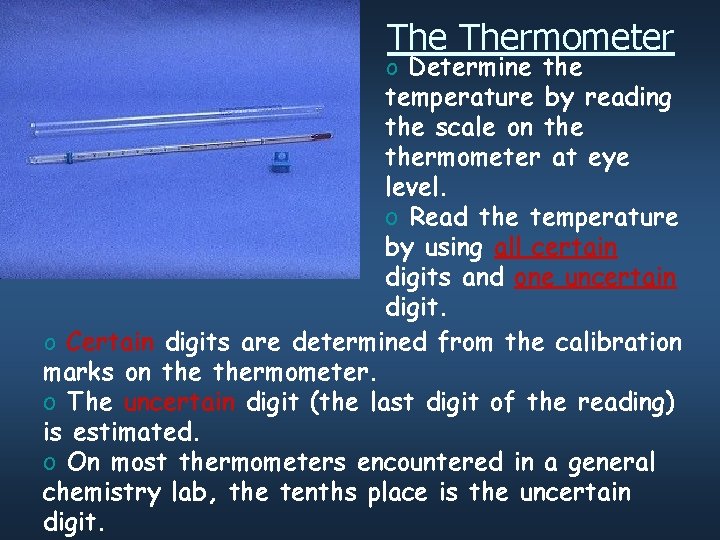
The Thermometer o Determine the temperature by reading the scale on thermometer at eye level. o Read the temperature by using all certain digits and one uncertain digit. o Certain digits are determined from the calibration marks on thermometer. o The uncertain digit (the last digit of the reading) is estimated. o On most thermometers encountered in a general chemistry lab, the tenths place is the uncertain digit.

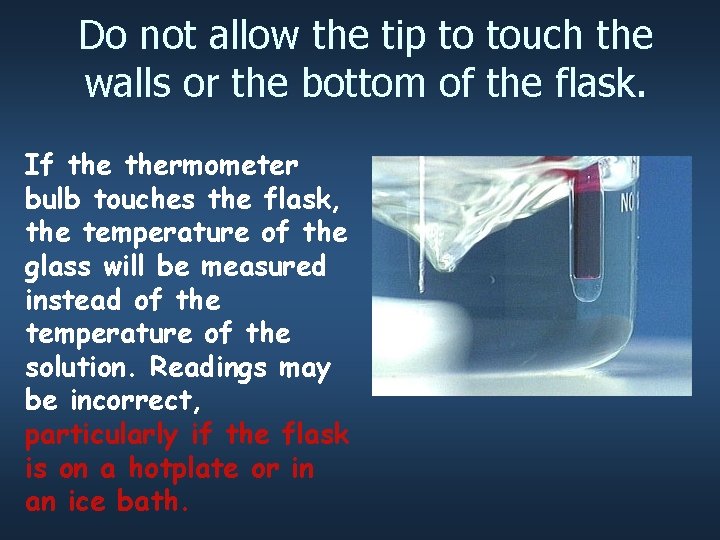
Do not allow the tip to touch the walls or the bottom of the flask. If thermometer bulb touches the flask, the temperature of the glass will be measured instead of the temperature of the solution. Readings may be incorrect, particularly if the flask is on a hotplate or in an ice bath.

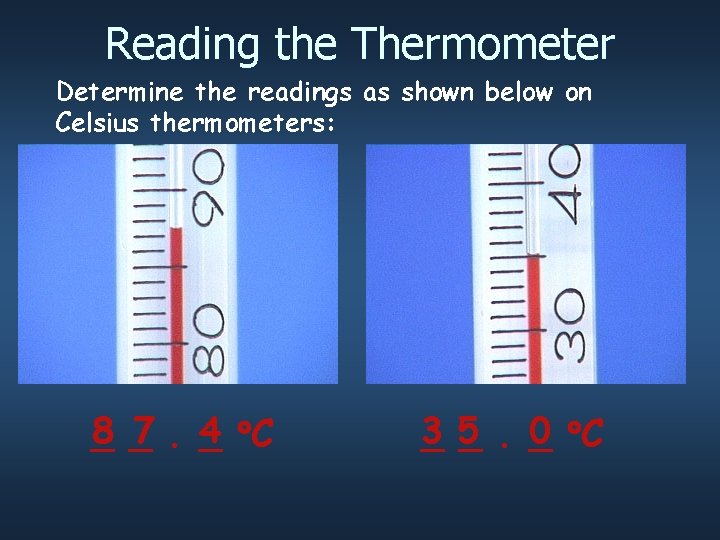
Reading the Thermometer Determine the readings as shown below on Celsius thermometers: 8 _ 7. _ 4 C _ 3 0 C _5 _. _

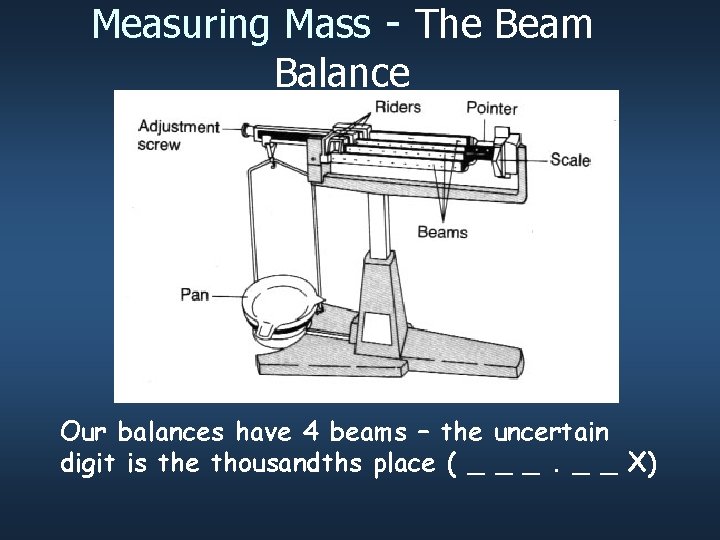
Measuring Mass - The Beam Balance Our balances have 4 beams – the uncertain digit is the thousandths place ( _ _ _ X)

Balance Rules In order to protect the balances and ensure accurate results, a number of rules should be followed: Ø Always check that the balance is level and zeroed before using it. Ø Never weigh directly on the balance pan. Always use a piece of weighing paper to protect it. Ø Do not weigh hot or cold objects. Ø Clean up any spills around the balance immediately.

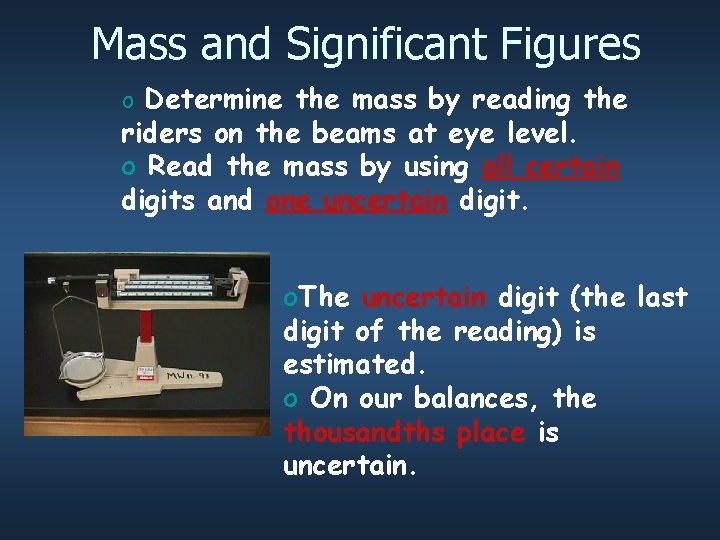
Mass and Significant Figures o Determine the mass by reading the riders on the beams at eye level. o Read the mass by using all certain digits and one uncertain digit. o. The uncertain digit (the last digit of the reading) is estimated. o On our balances, the thousandths place is uncertain.

Determining Mass 1. Place object on pan 2. Move riders along beam, starting with the largest, until the pointer is at the zero mark

Check to see that the balance scale is at zero

1 _ 4 _. _? _? ? _ Read Mass


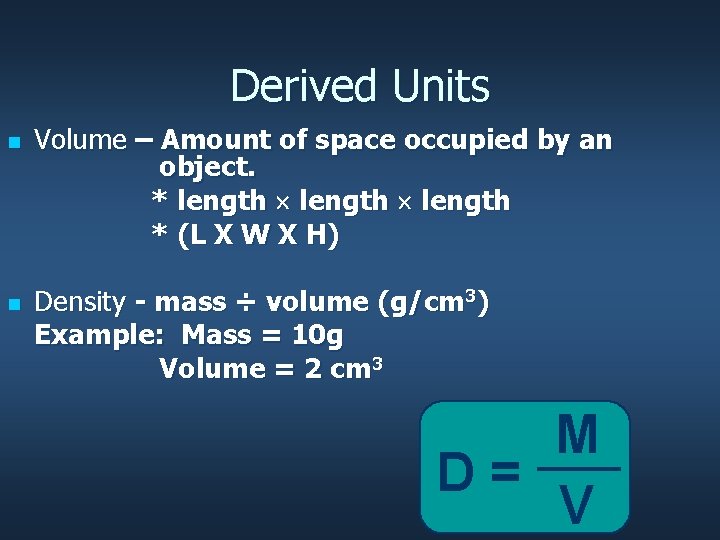
Derived Units n n Volume – Amount of space occupied by an object. * length * (L X W X H) Density - mass ÷ volume (g/cm 3) Example: Mass = 10 g Volume = 2 cm 3 M D= V

Density n An object has a volume of 825 cm 3 and a density of 13. 6 g/cm 3. Find its mass. GIVEN: WORK: V = 825 cm 3 D = 13. 6 g/cm 3 M=? M = DV M = (13. 6 g/cm 3)(825 cm 3) M = 11, 220 g

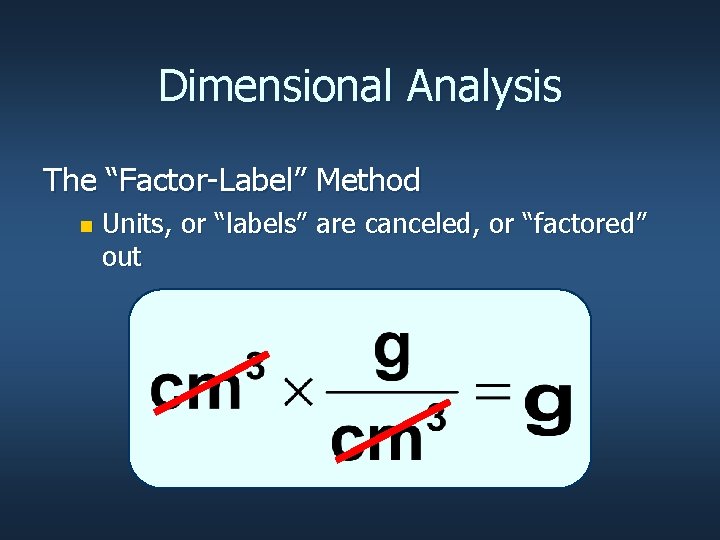
Dimensional Analysis The “Factor-Label” Method n Units, or “labels” are canceled, or “factored” out

Density 1) A liquid has a density of 0. 87 g/m. L. What volume is occupied by 25 g of the liquid? GIVEN: WORK: D = 0. 87 g/m. L V=? M = 25 g V= M D V= 25 g 0. 87 g/m. L V = 28. 7 m. L

Density 2) You have a sample with a mass of 620 g & a volume of 753 cm 3. Find density. GIVEN: WORK: M = 620 g V = 753 cm 3 D=? D=M V D= 620 g 753 cm 3 D = 0. 82 g/cm 3

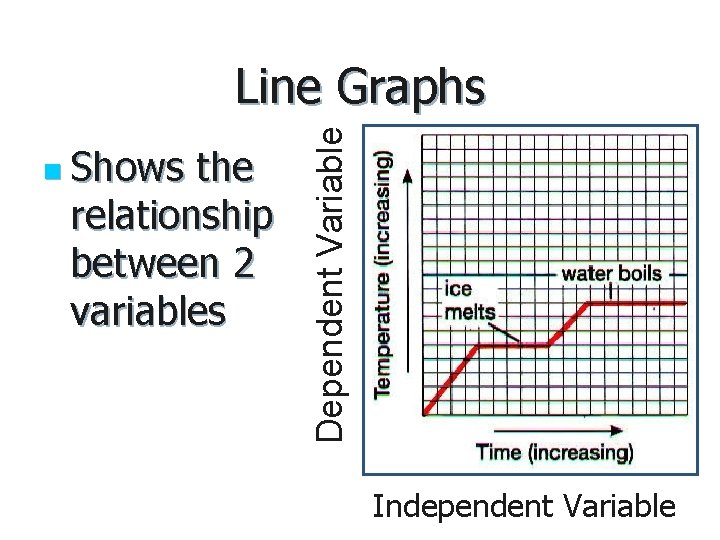
Creating Graphs

n Shows the relationship between 2 variables Dependent Variable Line Graphs Independent Variable

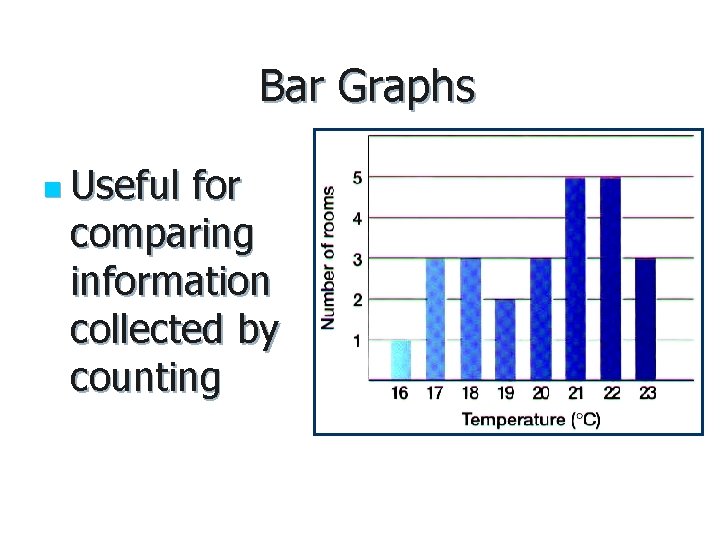
Bar Graphs n Useful for comparing information collected by counting

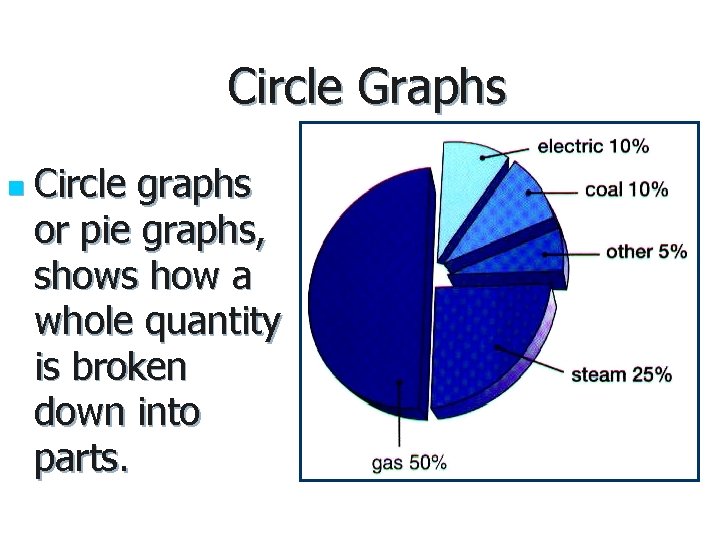
Circle Graphs n Circle graphs or pie graphs, shows how a whole quantity is broken down into parts.