Scientific Evaluation and Innovation in Risk Assessment approaches


























- Slides: 32

Scientific Evaluation and Innovation in Risk Assessment approaches in the area of Food Safety Promoting the leadership of agro-food industry Conference organised by the European Commission, 15 -16 November, Brussels Djien Liem Innovation in RA, EC Conference, Brussels, 16 Nov 2007 Scientific Committee & Advisory Forum Unit

Content of my presentation § EFSA – Where are we in 2007 • • Mandate Organisation, Partners and Networks Achievements Focus on Innovation in RA § Opportunities and future challenges Innovation in RA, EC Conference, Brussels, 16 Nov 2007 2

What EFSA does EFSA’s tasks 1. Provide scientific advice, opinions, information, and technical support for Community legislation and policies 2. Collect and analyse data to allow characterisation and monitoring of risks 3. Promote and coordinate development of uniform risk assessment methodologies 4. Communicate risks related to all aspects of EFSA’s mandate Innovation in RA, EC Conference, Brussels, 16 Nov 2007 3

What EFSA does What EFSA cannot do § Be responsible for food safety legislation § Take charge of food safety/quality controls, labelling or other such issues § Act as a substitute for national authorities Innovation in RA, EC Conference, Brussels, 16 Nov 2007 4

EFSA timeline Timeline 2002 Legal establishment (January) 1 st meeting of Management Board (September) 2003 Council decision establishes Parma as EFSA’s official seat (Dec) Scientific Committee and Panels begin work (May) 1 st meeting of Advisory Forum (March) 2004 Launch of EFSA website (March) 2005 Inauguration of EFSA’s official seat in Parma (June) 2006 Renewal of Management Board, Scientific Committee and Panels (June) Catherine Geslain-Lanéelle, Executive Director of EFSA (July) Over 200 support staff by end of 2006 Innovation in RA, EC Conference, Brussels, 16 Nov 2007 5

EFSA timeline EFSA today Where are we in 2007? Based in Parma, Italy Over 190 scientific experts (SC and Panels) Over 450 scientific opinions Over 140 press releases/statements EFSA Stakeholder Consultative Platform fully established § 10 Scientific Colloquia on key topics § Budget: € 51. 6 million for 2007 § § § Innovation in RA, EC Conference, Brussels, 16 Nov 2007 6

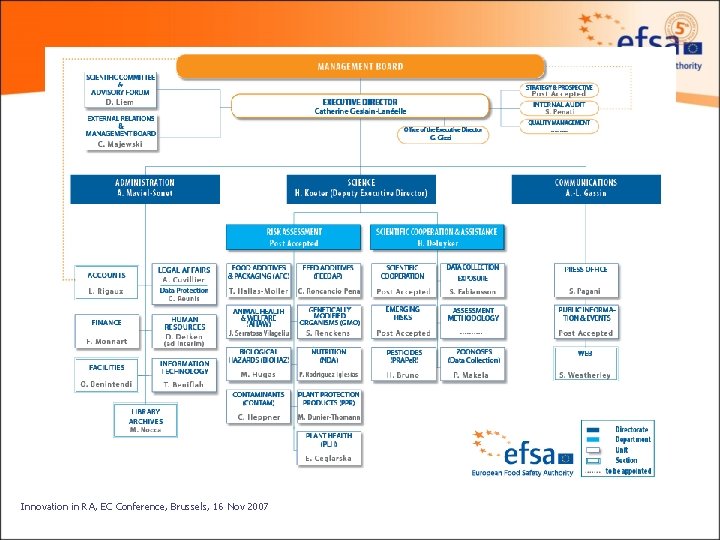
EFSA structure Management Board EFSA Directorate and Staff Innovation in RA, EC Conference, Brussels, 16 Nov 2007 Advisory Forum Scientific Committee and Panels 7

Management Board Role § Primary role: ensure Authority functions effectively and efficiently § Establish budget, agree work programmes and monitor implementation § Ensure Authority stays within remit of Founding Regulation § Appoint Executive Director, Scientific Committee and Panels § Audit Authority’s operations Innovation in RA, EC Conference, Brussels, 16 Nov 2007 8

Management Board Six Priorities June 2006 recommendations 1. Develop active networking and stronger cooperation with Member States 2. Strengthen EFSA’s relationship with its institutional partners (EU and international) and stakeholders 3. Enhance EFSA’s organisation 4. Enhance the impact and effectiveness of EFSA’s communications 5. Develop EFSA’s role in nutrition 6. Define EFSA’s medium and long-term vision Innovation in RA, EC Conference, Brussels, 16 Nov 2007 9

Advisory Forum Role § Advise EFSA on scientific matters, work programme/priorities and emerging risks § Ensure close collaboration between national bodies and EFSA § Assist in resolving contentious scientific issues and avoiding divergent views on food/feed safety issues § Avoid duplication of scientific effort § Play a key role in sharing and disseminating information (Declaration of Intent, Sep 2006) § Assist in increasing scientific co-operation between Member States (Strategy for Cooperation & Networking, Dec 2006) Innovation in RA, EC Conference, Brussels, 16 Nov 2007 10

EFSA Staff Overall profile § § 277 staff on 1 Sep 2007 Expected to reach 300 by end of 2007 Genuinely multinational and multicultural Temporary agents (236), contract agents (22), national experts (9) Innovation in RA, EC Conference, Brussels, 16 Nov 2007 11

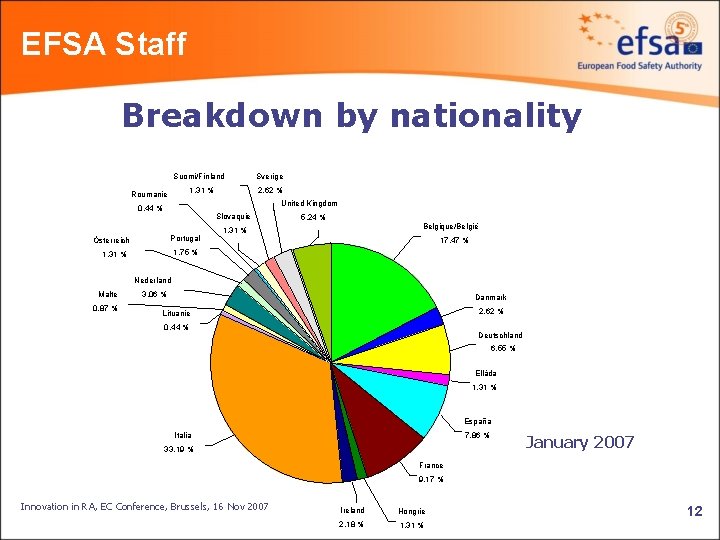
EFSA Staff Breakdown by nationality Suomi/Finland 1. 31 % Roumanie Sverige 2. 62 % United Kingdom 0. 44 % Slovaquie Portugal Österreich 5. 24 % Belgique/België 1. 31 % 17. 47 % 1. 75 % 1. 31 % Nederland Malte 0. 87 % 3. 06 % Danmark 2. 62 % Lituanie 0. 44 % Deutschland 6. 55 % Elláda 1. 31 % España Italia 7. 86 % 33. 19 % January 2007 France 9. 17 % Innovation in RA, EC Conference, Brussels, 16 Nov 2007 Ireland Hongrie 2. 18 % 1. 31 % 12

EFSA Staff Structure 3 Directorates § Science § Communications § Administration Science Directorate has 2 departments § Risk Assessment: Scientific Panels § Scientific Co-operation and Assistance Innovation in RA, EC Conference, Brussels, 16 Nov 2007 13

Innovation in RA, EC Conference, Brussels, 16 Nov 2007

Science Directorate Department of Scientific Cooperation and Assistance (SCA) comprising: Ø Data collection and exposure (DATEX) Unit; Ø Scientific cooperation (SCOOP) Unit; Ø Emerging risks (EMRISK) Unit; Ø Assessment methodology (ASMET) Unit; Ø Pesticides risk assessment (PRAPe. R) Unit; Ø Zoonosis Unit. Innovation in RA, EC Conference, Brussels, 16 Nov 2007 15

Science Scientific Committee and Panels Nine Scientific Panels* First established June 2003 - re-established June 2006 New Panel on Plant Heath created June 2006 Independent scientists selected on basis of proven excellence in their field § Mandatory commitment of independence § Declaration of Interest (annual and per meeting) § § * Split of AFC Panel into two Panels in preparation Innovation in RA, EC Conference, Brussels, 16 Nov 2007 16

The 9 Scientific Panels • Food additives, flavourings, processing aids, materials in contact with food (AFC) • Animal Health and Welfare (AHAW) • Biological hazards (BIOHAZ) • Contaminants in the food chain (CONTAM) • Additives and products in animal feed (FEEDAP) • Plant Protection Products (PPR) • Genetically modified organisms (GMO) • Dietetic products, nutrition and allergies (NDA) • Plant Health Panel (PLH) Innovation in RA, EC Conference, Brussels, 16 Nov 2007 17

Scientific Committee • Comprises the Chairs of all 9 Panels; • Additional 6 independent members; • Provides guidance to all Panels; • Manages projects involving several Panels; • Advises EFSA on emerging issues and priorities for scientific work. Innovation in RA, EC Conference, Brussels, 16 Nov 2007 18

Scientific activities (work themes): § General Requests for Scientific Opinions and Advice: providing scientific opinions, guidance and advice in response to questions; § Authorisations: Assessing the risk of regulated substances and development of proposals for risk-related factors; § Monitoring and assessing specific biological risk factors for human animal health and animal diseases: BSE/TSE, Zoonoses, plant health Ø Improving the European risk assessment approaches and methodologies: Development, promotion and application of new and harmonized scientific approaches and methodologies for hazard and risk assessment Innovation in RA, EC Conference, Brussels, 16 Nov 2007 19

Harmonisation/Innovation 2003 -2007 § Guidance on RA approaches to be used by EFSA’s Panels and Expert working groups • Guidance documents on RA approaches in the area of GMOs, Animal Nutrition, Plant Protection Products • Harmonised methods for Exposure Assessment (e. g. how to organise EA in EFSA, handling new trends and developments, advice on how to access relevant data, uncertainties and variability) • Harmonised approach for RA of substances that are both Genotoxic and Carcinogenic • Qualified Presumption of Safety: a generic approach for the safety assessment of microorganisms in food/feed Innovation in RA, EC Conference, Brussels, 16 Nov 2007 20

New and harmonised RA: Ongoing and planned activities AMONG OTHERS: § Transparency in RA – Part 2: Science-related aspects § Welfare of experimental animals § Benchmark Dose Approach § Botanicals and botanical preparations § Qualified Presumption of Safety § Risk-Benefit assessments § RA of application of nanoscience and -technology in food/feed Innovation in RA, EC Conference, Brussels, 16 Nov 2007 21

New and harmonised RA: Ongoing and planned activities AMONG OTHERS: (cont’d) § Applicability of the TTC concept in EFSA’s risk assessments § Follow-up of SC’s opinions on genotoxic carcinogens and exposure assessment § Quantitative Microbiological RA and § Various scientific cooperation projects Innovation in RA, EC Conference, Brussels, 16 Nov 2007 22

Science Origin of requests for 2006 Innovation in RA, EC Conference, Brussels, 16 Nov 2007 23

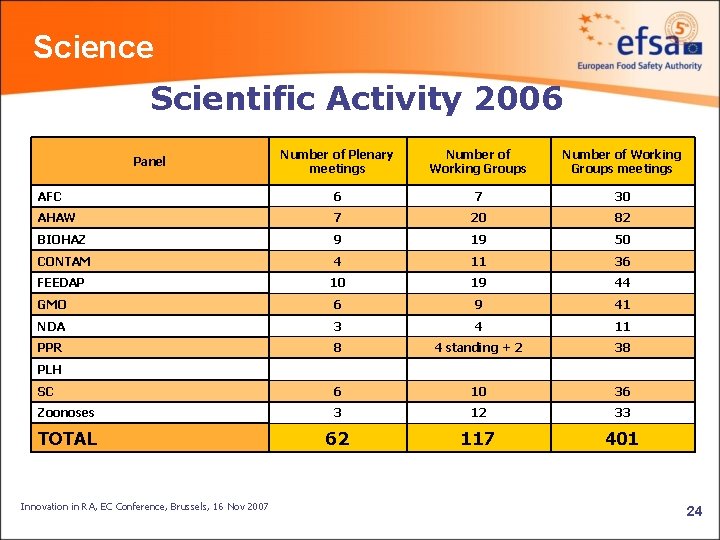
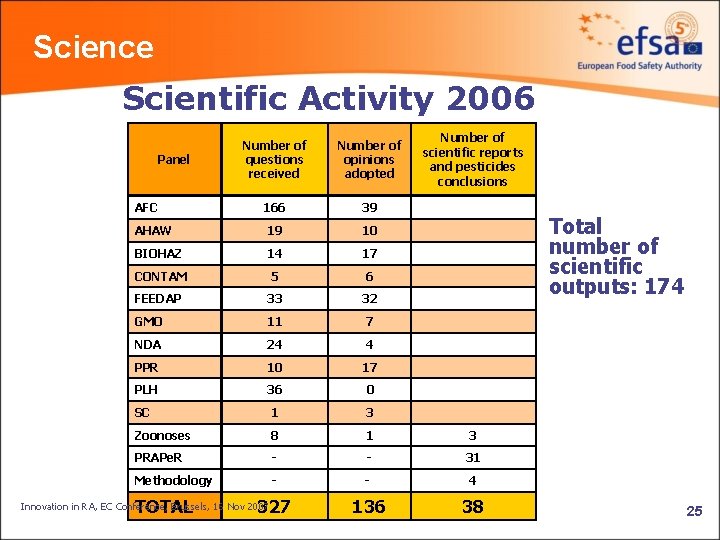
Science Scientific Activity 2006 Number of Plenary meetings Number of Working Groups meetings AFC 6 7 30 AHAW 7 20 82 BIOHAZ 9 19 50 CONTAM 4 11 36 FEEDAP 10 19 44 GMO 6 9 41 NDA 3 4 11 PPR 8 4 standing + 2 38 SC 6 10 36 Zoonoses 3 12 33 TOTAL 62 117 401 Panel PLH Innovation in RA, EC Conference, Brussels, 16 Nov 2007 24

Science Scientific Activity 2006 Number of scientific reports and pesticides conclusions Number of questions received Number of opinions adopted 166 39 AHAW 19 10 BIOHAZ 14 17 CONTAM 5 6 FEEDAP 33 32 GMO 11 7 NDA 24 4 PPR 10 17 PLH 36 0 SC 1 3 Zoonoses 8 1 3 PRAPe. R - - 31 Methodology - - 4 327 136 38 Panel AFC TOTAL Innovation in RA, EC Conference, Brussels, 16 Nov 2007 Total number of scientific outputs: 174 25

Science Scientific Colloquia 1. 2. 3. 4. 5. 6. 7. Dioxins: June 2004, Brussels Qualified Presumption of Safety: December 2005, Brussels Food Consumption: April 2005, Brussels Animal Welfare: December 2005, Parma Food-based Dietary guidelines: March 2006, Parma Risk-Benefit Analysis of Foods: July 2006, Tabiano Pesticides Cumulative Risk Assessment : November 2006, Parma 8. Environmental RA of GM Plants: June 2007, Tabiano 9. Nutrient Profiling and Health Claims: October 2007, Parma 10. RA in Plant Health: December 2007, Parma Innovation in RA, EC Conference, Brussels, 16 Nov 2007 26

Looking to the future Vision “My vision is for EFSA to become globally recognised as the European reference body for risk assessment on food and feed safety, animal health and welfare, nutrition, plant protection and plant health” Catherine Geslain-Lanéelle, Executive Director of EFSA Innovation in RA, EC Conference, Brussels, 16 Nov 2007 27

Looking to the future Future challenges and perspectives § Handling the Workload (prioritisation) § Credibility of scientific advice § New areas of work: – – Nanotechnology Animal cloning Claims … § Scientific co-operation with Member States (focal points) § International relations strategy § Expand consolidate EFSA organisation Innovation in RA, EC Conference, Brussels, 16 Nov 2007 28

Priority areas for scientific cooperation (EFSA, 2006) § Exchanging and collecting scientific data and information § Sharing best risk assessment practices § Developing harmonized methodologies for risk assessment § Promoting coherence in risk communications Innovation in RA, EC Conference, Brussels, 16 Nov 2007 29

Scientific cooperation projects (Nov 2007) § Establishing a European network of managers of chemical occurrence data § Establishing a European network of food consumption database managers § § Risks and benefit of the fortification of food with folic acid Horizon scanning to identify emerging food safety risks Setting-up a database of national experts in Europe Botanicals and botanical preparations § Harmonisation of risk assessment approaches in Member States Innovation in RA, EC Conference, Brussels, 16 Nov 2007 30

Looking to the future (Cont’d) Future challenges and perspectives § § § Data collection (chemical and biological hazards, dietary exposure, consumer choice) Harmonising methodologies for RA across Europe Building (together) a system for identification and evaluation of emerging risks Implementing preparedness tools for predictable/unpredictable events Governance/Confidence/Changing society/Globalisation (key challenges SANCO 2009 -2014) Innovation in RA, EC Conference, Brussels, 16 Nov 2007 31

Thanks for your kind attention !! Innovation in RA, EC Conference, Brussels, 16 Nov 2007