Science Systems Matter and Energy Ch 2 A





















- Slides: 51

Science, Systems, Matter, and Energy Ch 2

A. The Nature of Science 1. Science assumes that events in the natural world follow orderly patterns and that these patterns can be understood 2. Based on observations of phenomenon, scientists form a scientific hypothesis a) Hypothesis — an unconfirmed explanation of an observed phenomenon to be tested 3. A scientific theory is a verified, believable, widely accepted scientific hypothesis or a related group of scientific hypotheses

a) Theories are the most reliable knowledge we have about how nature works 4. A scientific/natural law describes events/actions of nature that reoccur in the same way, over and over again a) They always include a degree of uncertainty. 5. Scientists use both inductive reasoning and deductive reasoning a) Inductive reasoning uses specific observations and measurements to arrive at a conclusion b) Deductive reasoning uses logic to arrive at a specific conclusion

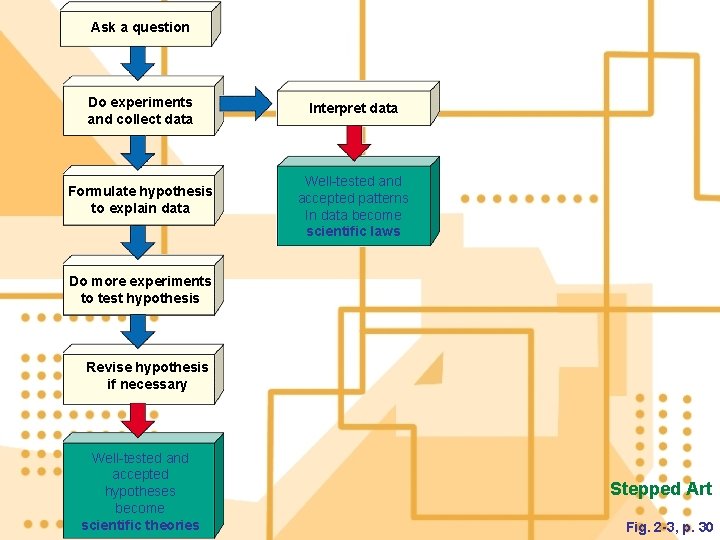
Ask a question Do experiments and collect data Formulate hypothesis to explain data Interpret data Well-tested and accepted patterns In data become scientific laws Do more experiments to test hypothesis Revise hypothesis if necessary Well-tested and accepted hypotheses become scientific theories Stepped Art Fig. 2 -3, p. 30

6. Frontier science is scientific results that have not been confirmed; 7. Sound science results from scientific results that have been well tested and are widely accepted. 8. Media people mislead us, consider the reliability of the individuals presenting the data.

B. Models and Behavior of Systems 1. Scientists project the behavior of complex systems by developing a model of its inputs, throughputs (flows), and outputs of matter, energy, and information. a) Mathematical models consist of one or a series of equations used to describe behavior of a system (e. g. Hurricane behavior)

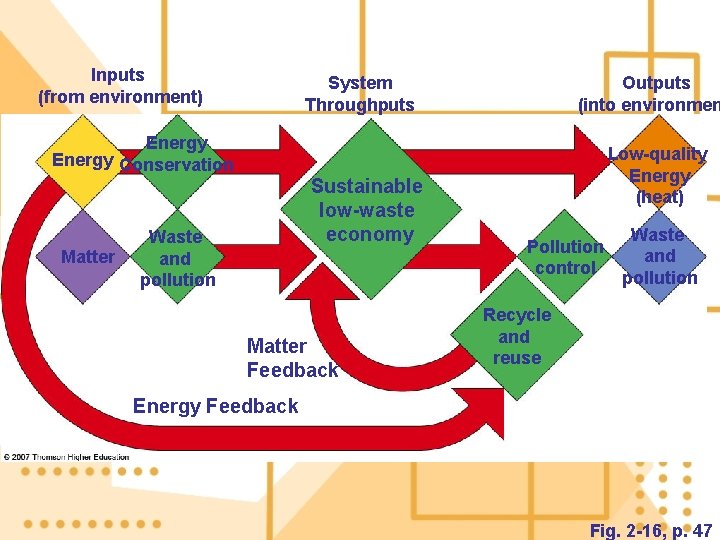
Inputs (from environment) System Throughputs Outputs (into environmen Energy Conservation Matter Sustainable low-waste economy Waste and pollution Matter Feedback Low-quality Energy (heat) Pollution control Waste and pollution Recycle and reuse Energy Feedback Fig. 2 -16, p. 47

2. Feedback loops can cause a system to do more positive feedback (what it was doing) or negative feedback (in opposite direction)

Animation: Feedback Control of Temperature PLAY ANIMATION

3. A synergistic interaction results in the combined effects interact to amplify the results a) smoking magnify the effect of asbestos exposure on lung cancer

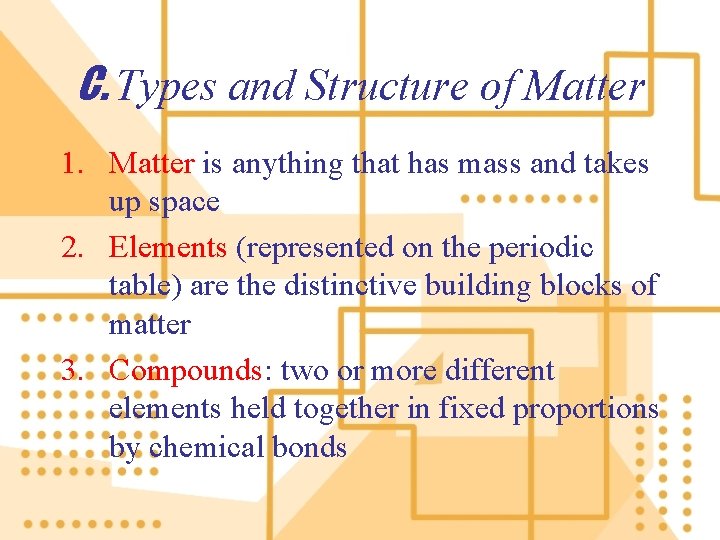
C. Types and Structure of Matter 1. Matter is anything that has mass and takes up space 2. Elements (represented on the periodic table) are the distinctive building blocks of matter 3. Compounds: two or more different elements held together in fixed proportions by chemical bonds

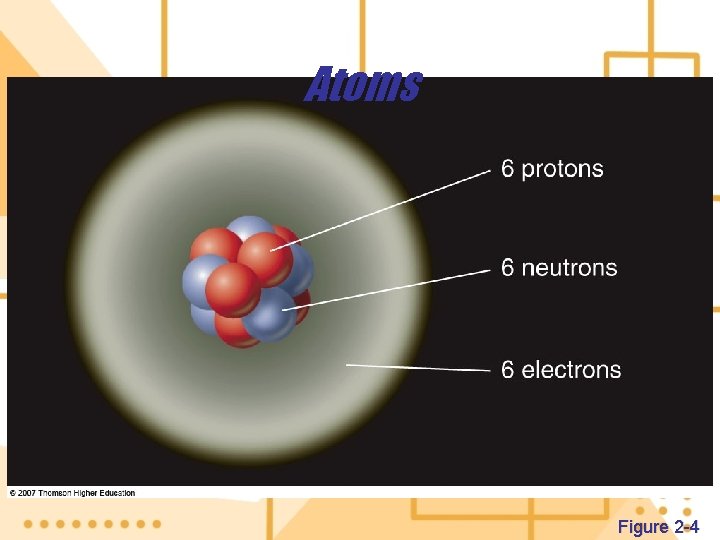
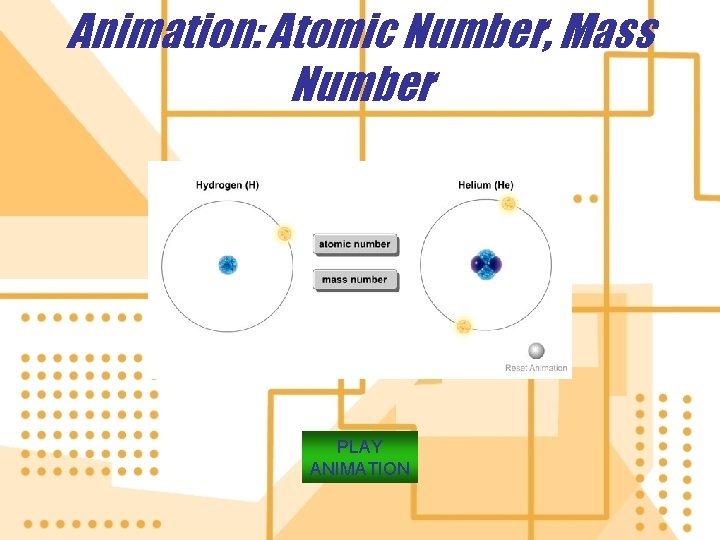
4. The building blocks of matter are atoms a) Each atom has a nucleus containing protons and neutrons and one or more electrons orbit the nucleus. b) A proton (p) is positively charged. c) A neutron (n) is uncharged. d) The electron (e) is negatively charged 5. Isotopes of an element are various forms of an element that have the same atomic number, but different mass number.

Atoms Figure 2 -4

Animation: Subatomic Particles PLAY ANIMATION

Animation: Atomic Number, Mass Number PLAY ANIMATION

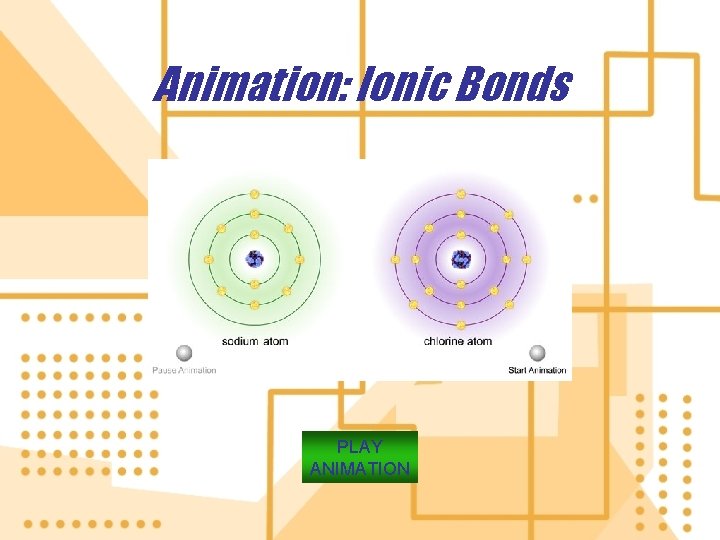
6. An ion is an atom or group of atoms with one or more net positive or negative electrical charges. a) e. g. Hydrogen ions (H+), Hydroxide ions (OH- ) b) Elements known, as metals tend to lose one or more electrons c) Elements known, as nonmetals tend to gain more electrons

Animation: Ionic Bonds PLAY ANIMATION

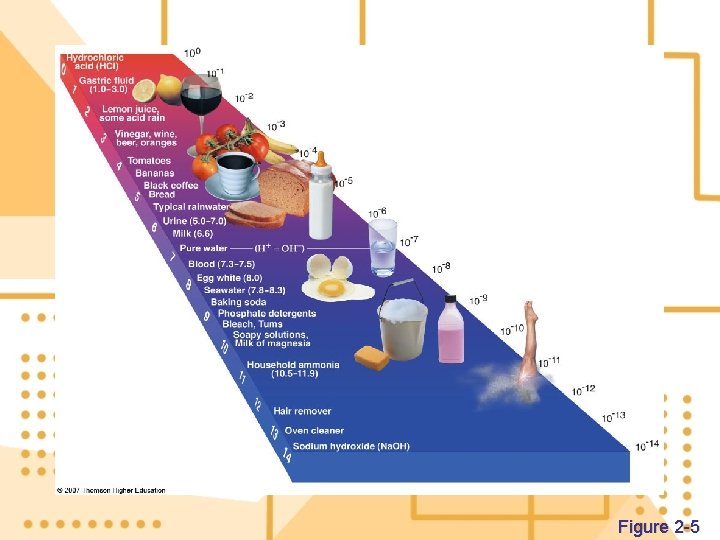
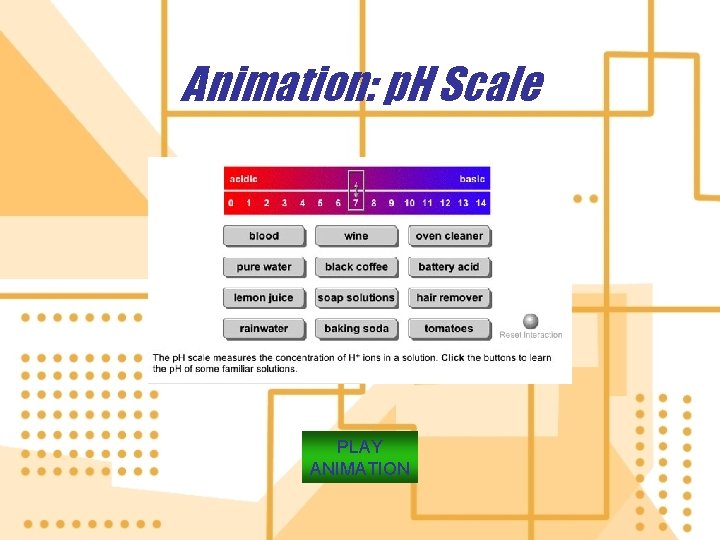
7. Hydrogen ions (H+) in a solution are a measure of how acidic or basic the solution a) The amount of a substance in a unit volume of air, water, or other medium is its concentration b) The p. H (potential of Hydrogen) is the concentration of hydrogen ions in one liter of solution

Figure 2 -5

Animation: p. H Scale PLAY ANIMATION

8. Chemical formulas are a type of shorthand to show the type and number of atoms/ions in a compound a) Ionic compounds are made up of oppositely charged ions, (Na+ and Cl-). b) Compounds made of uncharged atoms are called covalent compounds (CH 4).

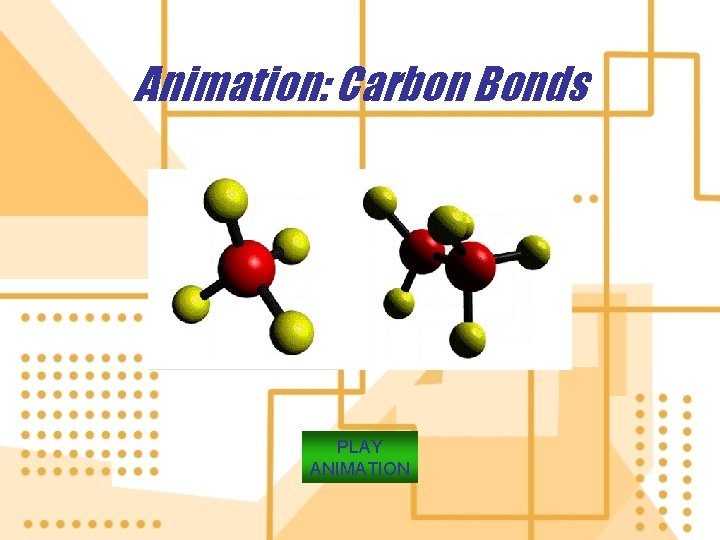
9. Organic compounds contain carbon atoms combined with one another and with various other atoms such as H+, N+, or Cl-. a) Contain at least two carbon atoms combined with each other and with atoms. b) Methane (CH 4) is the only exception. c) All other compounds are inorganic. d) Hydrocarbons: compounds of carbon and hydrogen atoms e) Chlorinated hydrocarbons: compounds of carbon, hydrogen, and chlorine atoms 9. Simple carbohydrates: specific types of compounds of carbon, hydrogen and oxygen atoms. (e. g. sugars)

Animation: Carbon Bonds PLAY ANIMATION

10. Large, complex organic molecules (macromolecules) make up the basic molecular units found in living organisms; four major types: a) Complex carbohydrates contain two or more monomers of simple sugars b) Proteins are formed by linking monomers of amino acids c) Nucleic acids are made of sequences of nucleotides d) Lipids large organic compounds that are nonpolar ex; fats, oils, steroids, and hormones.

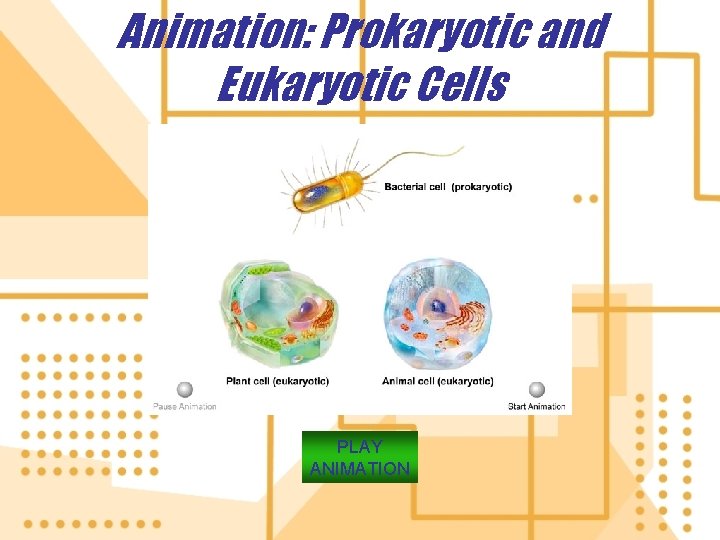
11. All living things are composed of cells 1. Cells of eukaryotic organisms have a membrane, nucleus, and organelles 2. Cells of prokaryotic organisms have a membrane but no defined nucleus or organelles 3. Organic molecules are the building blocks of life

Animation: Prokaryotic and Eukaryotic Cells PLAY ANIMATION

12. Matter exists in four states, solid, liquid, gaseous and as plasma a) Water can exist in all three forms b) Plasma is a high-energy mixture of positively charged ions and negatively charged electrons. c) Scientists make artificial plasmas in fluorescent lights, TV and computer screens.

13. Matter can be classified as having high or low quality depending on how useful it is to us as a resource. a) High quality matter is concentrated and easily extracted. b) low quality matter is more widely dispersed and more difficult to extract. Figure 2 -8

c) Material efficiency/resource productivity describes the total amount of material needed to produce a unit of good/service.

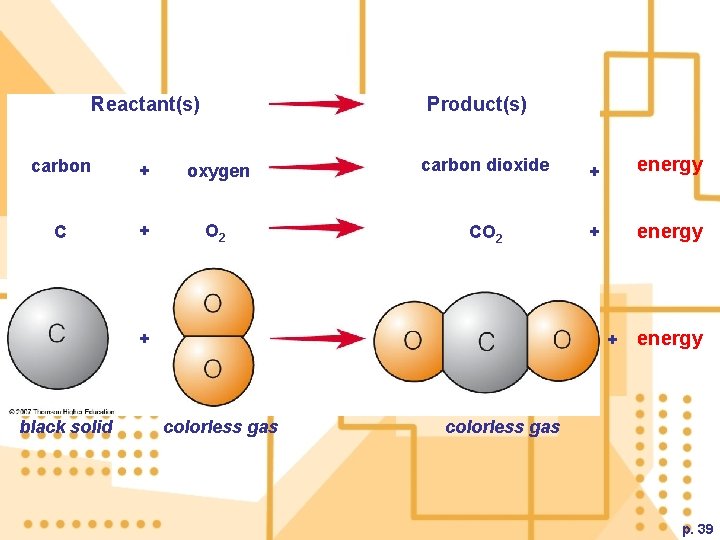
D. Changes in Matter 1. The Law of conservation of matter states that no atoms are created/destroyed during a physical or chemical change a) In a physical change, the molecules are organized in different patterns. b) In a chemical change, the chemical composition of the elements/compounds change c) Chemical equations are used to verify that no atoms are created or destroyed in a chemical reaction

Changes in Matter

Reactant(s) Product(s) carbon + oxygen carbon dioxide + energy C + O 2 CO 2 + energy + black solid + colorless gas energy colorless gas p. 39

2. We will always have some pollutants, but can produce less and clean up some that we do produce 3. Factors that determine the severity of a pollutant’s effects: chemical nature, concentration, and persistence. 4. Pollutants are classified based on their persistence: a) b) c) d) Degradable pollutants Biodegradable pollutants Slowly degradable pollutants Nondegradable pollutants

5. Three types of nuclear change are radioactive decay, nuclear fission, and nuclear fusion. 6. Radioactive decay is the change of a radioisotope to different isotope by spontaneously emitting fast moving chunks of matter a) The rate of decay is expressed as a half-life (the time needed for one-half of the nuclei to decay to form a different isotope) b) Exposure to ionizing radiation from this damages cells in two possible ways: genetic damage (mutation of DNA molecules), body cell damage to tissues.

Animation: Half-Life PLAY ANIMATION

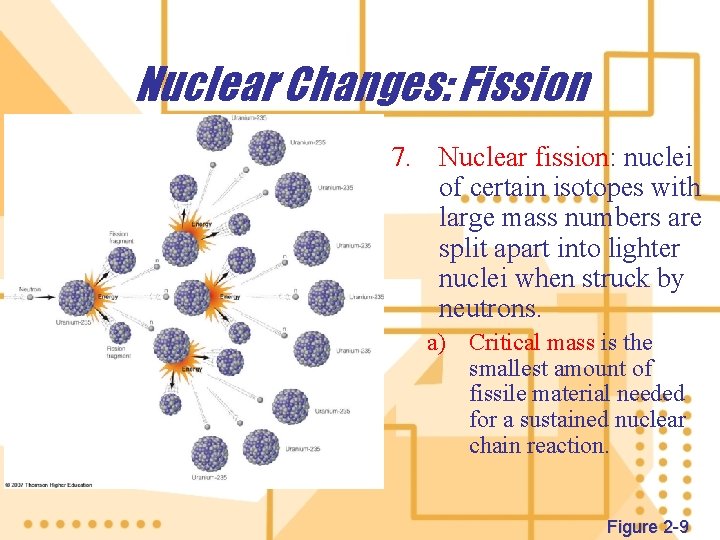
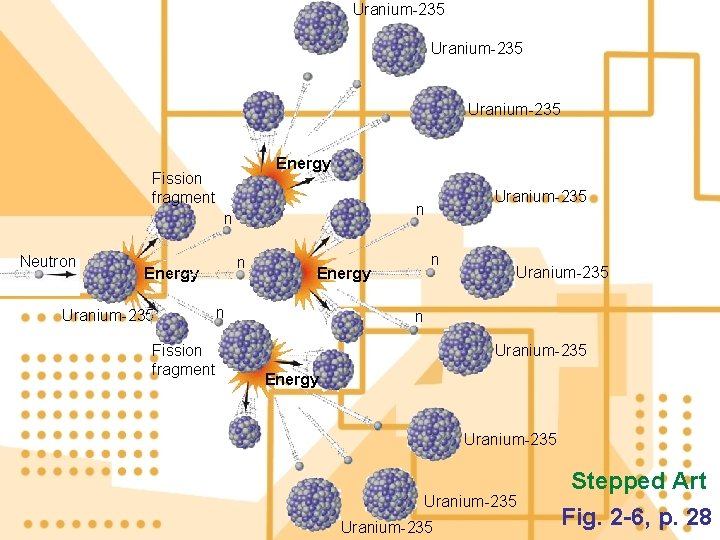
Nuclear Changes: Fission 7. Nuclear fission: nuclei of certain isotopes with large mass numbers are split apart into lighter nuclei when struck by neutrons. a) Critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. Figure 2 -9

Uranium-235 Energy Fission fragment n Neutron n Energy Uranium-235 Fission fragment Uranium-235 n n Energy n Uranium-235 Energy Uranium-235 Stepped Art Fig. 2 -6, p. 28

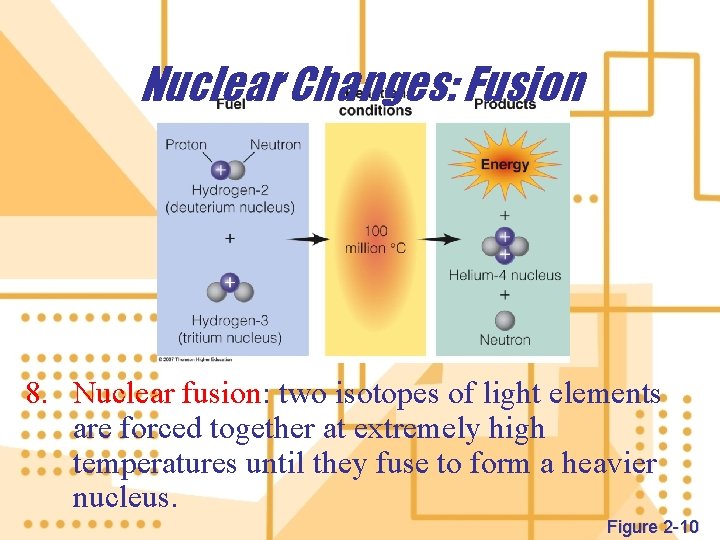
Nuclear Changes: Fusion 8. Nuclear fusion: two isotopes of light elements are forced together at extremely high temperatures until they fuse to form a heavier nucleus. Figure 2 -10

Video: Nuclear Energy PLAY VIDEO • From ABC News, Environmental Science in the Headlines, 2005 DVD.

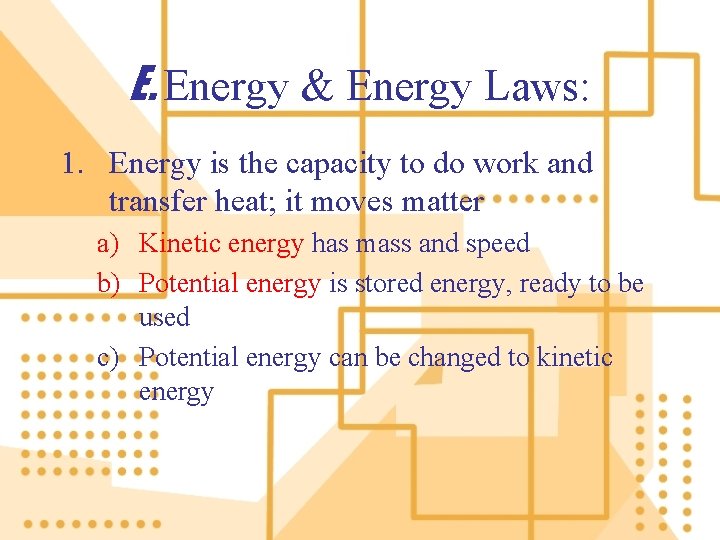
E. Energy & Energy Laws: 1. Energy is the capacity to do work and transfer heat; it moves matter a) Kinetic energy has mass and speed b) Potential energy is stored energy, ready to be used c) Potential energy can be changed to kinetic energy

Animation: Martian Doing Mechanical Work PLAY ANIMATION

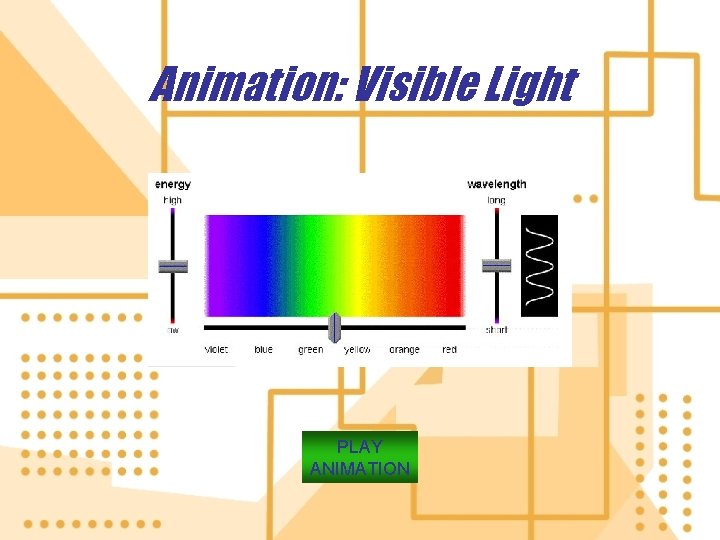
2. Electromagnetic radiation is energy that travels as a wave, a result of changing electric and magnetic fields a) The electromagnetic spectrum describes the range of electromagnetic waves that have different wavelengths and energy content b) Organisms vary in there ability to sense different parts of the spectrum.

Original Energy Source

Solar ejection

Sun Ionizing radiation Far Cosmic. Gamma X rays ultraviolet rays Rays Nonionizing radiation Near Far ultra- Visibleinfrared violet Waves waves High energy, short Wavelength in meters (not to scale) Microwaves TV waves Radio Waves Low energy, long Wavelength Fig. 2 -11, p. 43

Animation: Visible Light PLAY ANIMATION

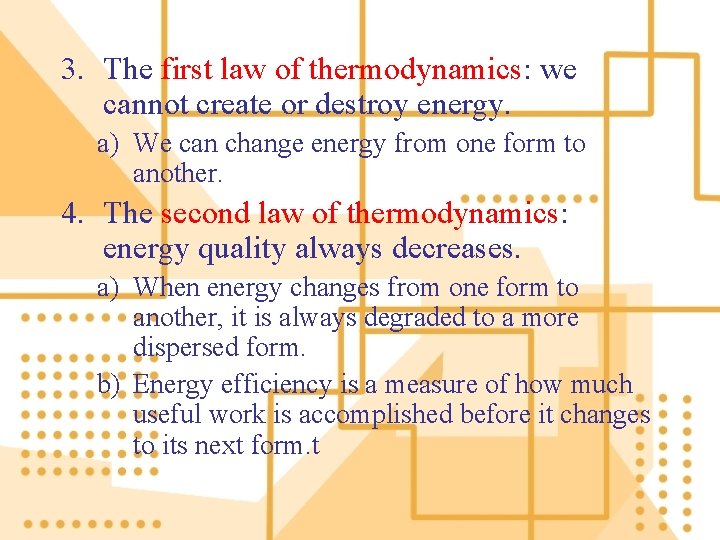
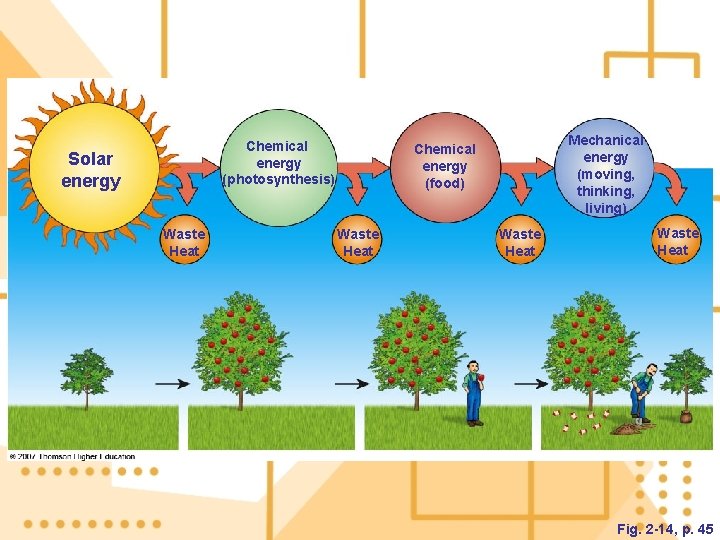
3. The first law of thermodynamics: we cannot create or destroy energy. a) We can change energy from one form to another. 4. The second law of thermodynamics: energy quality always decreases. a) When energy changes from one form to another, it is always degraded to a more dispersed form. b) Energy efficiency is a measure of how much useful work is accomplished before it changes to its next form. t

Animation: Total Energy Remains Constant PLAY ANIMATION

Chemical energy (photosynthesis) Solar energy Waste Heat Mechanical energy (moving, thinking, living) Chemical energy (food) Waste Heat Fig. 2 -14, p. 45

F Sustainability and Matter and Energy Laws 1. Resource use automatically adds some waste heat/waste to the environment 2. Advanced industrialized countries have high-throughput (high waste) economies a) Eventually consumption will exceed capacity of the environment to dilute/degrade wastes 3. Recycling and reusing more of earth’s matter resources slows depletion of nonrenewable resources,

4. Shifting to a more sustainable, lowthroughput (low-waste) economy is the best long-term solution to environmental/resource problems a) Waste less matter, live more simply, slow population growth.