SCIENCE STARTER WEEK 14 DAY 4 5516 7







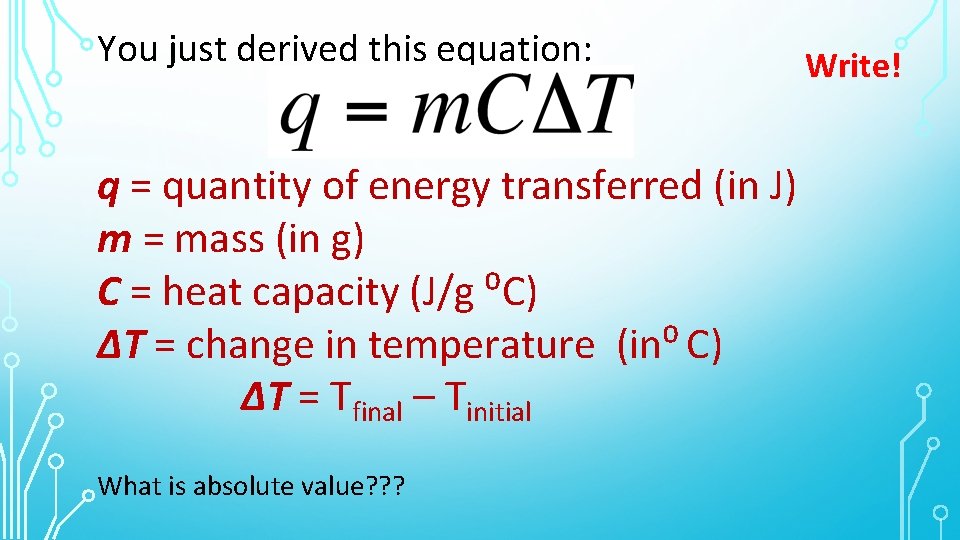






- Slides: 23

SCIENCE STARTER (WEEK 14, DAY 4, 5/5/16) 7. 1 Homework on desk! 1) Look at the heating curve learned about yesterday. Describe where phase energy is increasing and where thermal energy is increasing. 2) You have a tea kettle of water boiling on a stove. If you turn up the burner would the temperature of the water change? Explain your answer. 3) Put the following elements in order of increasing electronegativity. C, B, O, N

OBJECTIVE • SWBAT identify the parts of the heating and cooling curve • Understand the difference between thermal and phase energy • SWBAT describe heat capacity

AGENDA • Science Starter • Re-loop Yesterday • Demo: Dolla Billz • Identifying Heat Equations and Practice • Poster book Work Time

QUICK CHECK 1. When phase energy is increasing, temperature _________. 2. When thermal energy is increasing, phase energy ________. 3. What happens to temperature as I melt a block of ice? 4. What does a cooling curve look like?

WHY DOESN’T THE TEMPERATURE INCREASE DURING MELTING AND EVAPORATING? • HEAT is constant but the energy changes at a weird rate. • WHY? Because of the difference between thermal energy and phase energy. • Thermal energy changes temperature • Phase energy breaks down phases

UNIT 7: HEAT AND ENERGY SPECIFIC HEAT QUANTITATIVE ENERGY PROBLEMS

DEMO: DOLLAR BILLS • Background Info: • Law of Conservation of Energy: Energy is neither created nor destroyed, it is only transferred. • Eye Level: • Particle Level:

Notes DEMO: DOLLAR BILLS Consensus: Heat capacity (also called “specific heat”) = amount of energy required to raise the temperature of one gram of that substance by one degree Celsius • We measure energy in joules (J)

IT’S HAPPENED TO YOU: • It’s a hot summer day. You get into your car. The seatbelt is BURNING hot (ouch!) However, a bottle of water you left in the back seat is only slightly warm. WHY? ? !

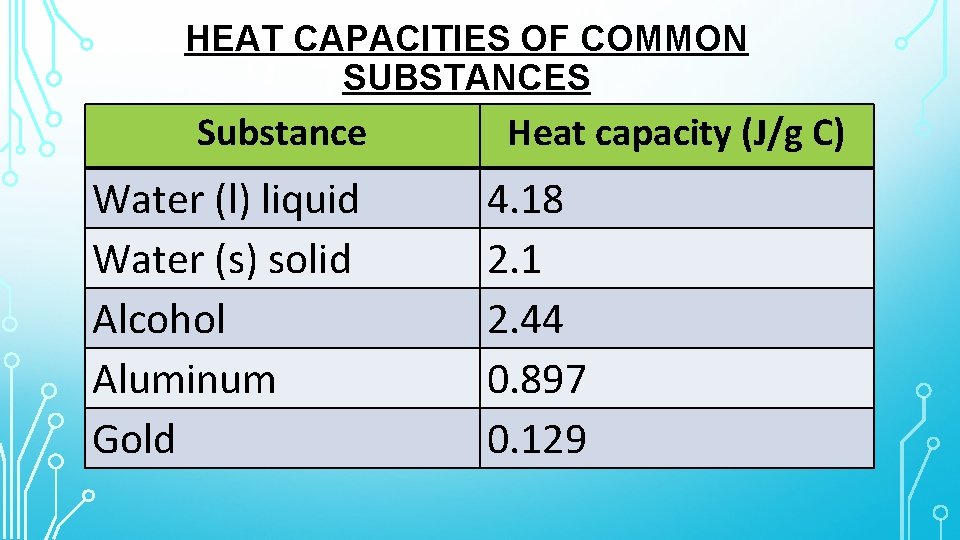
HEAT CAPACITIES OF COMMON SUBSTANCES Substance Water (l) liquid Water (s) solid Alcohol Aluminum Gold Heat capacity (J/g C) 4. 18 2. 1 2. 44 0. 897 0. 129

SPECIFIC HEAT LIKE SPECIFIC PAIN? Low Specific Pain: Ribs and other sensitive areas – LOW TOLERANCE FOR PAIN = Quicker Cringing! High Specific Pain: Arms and legs – HIGHER TOLERANCE FOR PAIN = Less Cringing!

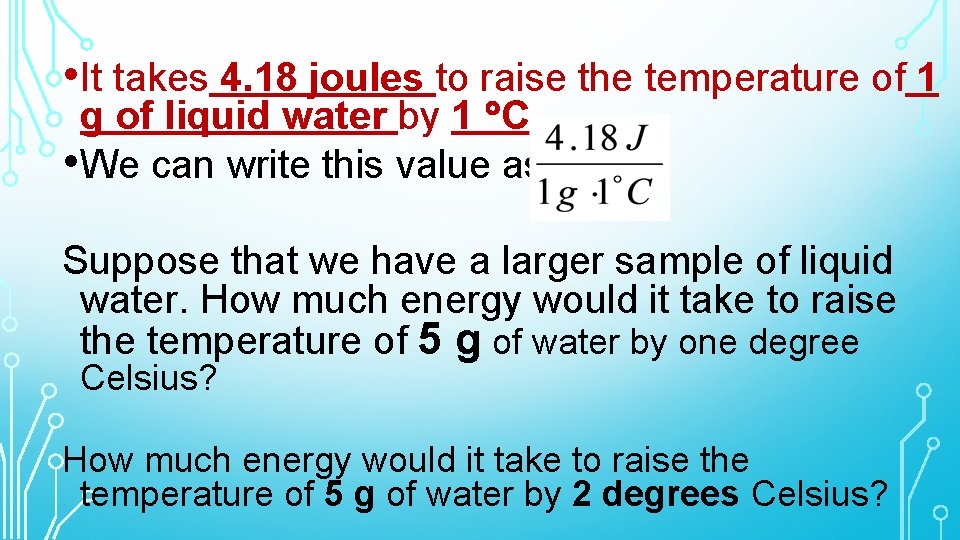
• It takes 4. 18 joules to raise the temperature of 1 g of liquid water by 1 C. • We can write this value as: Suppose that we have a larger sample of liquid water. How much energy would it take to raise the temperature of 5 g of water by one degree Celsius? How much energy would it take to raise the temperature of 5 g of water by 2 degrees Celsius?
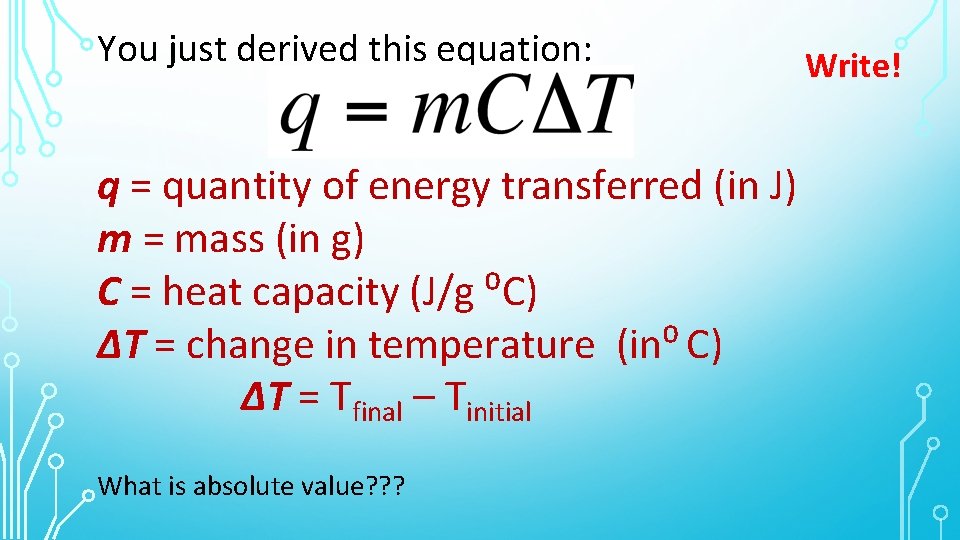
You just derived this equation: q = quantity of energy transferred (in J) m = mass (in g) C = heat capacity (J/g ⁰C) ∆T = change in temperature (in⁰ C) ∆T = Tfinal – Tinitial What is absolute value? ? ? Write!

How much energy does it take to raise the temperature of 5 g of water by 2 degrees celsius? How could we graph this on a heating/cooling curve?

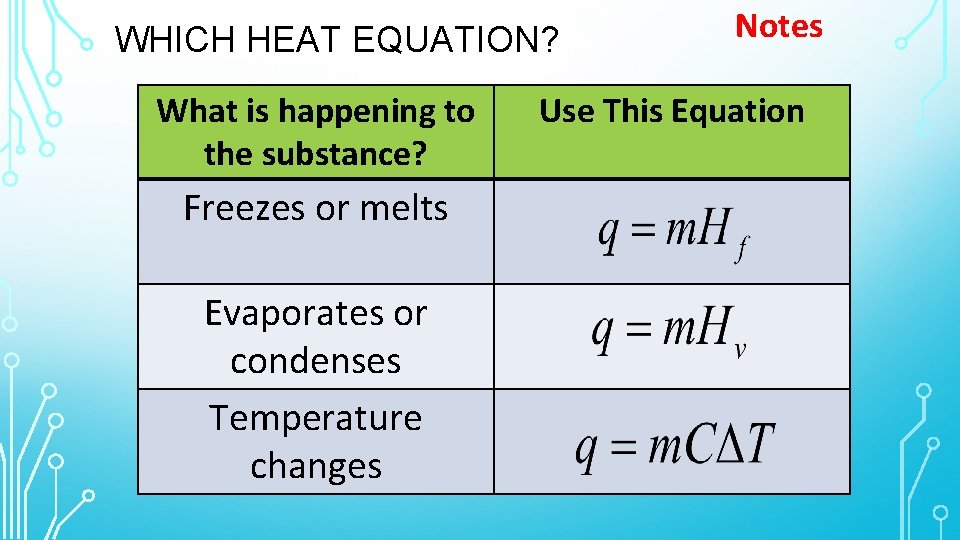
WHICH HEAT EQUATION? What is happening to the substance? Freezes or melts Evaporates or condenses Temperature changes Notes Use This Equation

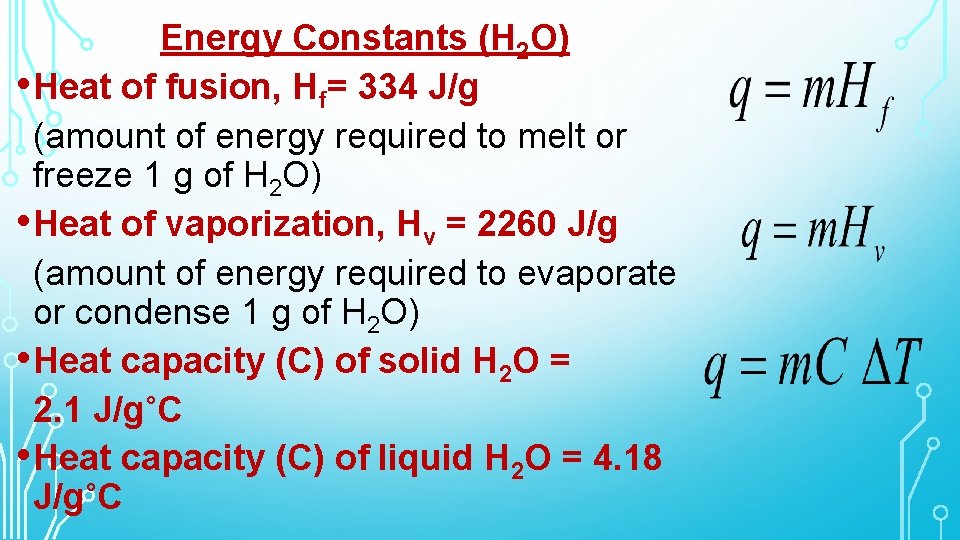
Energy Constants (H 2 O) • Heat of fusion, Hf= 334 J/g (amount of energy required to melt or freeze 1 g of H 2 O) • Heat of vaporization, Hv = 2260 J/g (amount of energy required to evaporate or condense 1 g of H 2 O) • Heat capacity (C) of solid H 2 O = 2. 1 J/g˚C • Heat capacity (C) of liquid H 2 O = 4. 18 J/g˚C

WAIT! WHICH EQUATION DO I USE? ? !! 1. A cup of hot chocolate (270 g) cools from 60˚C down to comfortable room temperature 20. ˚C. How much energy does it release to the surroundings? First, before you do any math, you need to determine what changes are taking place.

STEP S 1. What changes are taking place? (Is temperature changing? Is phase changing? ) 2. Which heat equation? 3. Identify variables. 4. Plug in and solve.

Another example on your worksheet… 2. A 110 g cup of water spills on your kitchen floor. If all of the water evaporates, how much energy did the water absorb from the surroundings? Identify info – Strategy (equation) –


PARTNER PRACTICE 3. You accidentally left your flavor-ice popsicle, with a mass of 43 g at 0˚C, on a park bench during the summer. By the time you got back from the basketball court it is totally melted. How much energy must be absorbed by the ice in order for it to melt all the way? (assume no temperature change) Identify info – Strategy (equation) –

TELL ME WHICH HEAT EQUATION AND WHY? 1. There is a 150 g puddle of water at 0 C outside. It freezes overnight. How much energy was required to freeze the water? 2. A 320 g cup of iced tea warms on a summer day from 10 degrees Celsius to 40 degrees Celsius. How much energy was required? 3. If q is positive, this means that heat was (lost)/(gained).

MINI POSTER BOOK WORK TIME • Use this time to work on your Semester Mini Poster Book (Units 1 -3 should be complete) • Instructions and materials are located at the front table