Science Starter HW ON DESK 1 What is













- Slides: 24

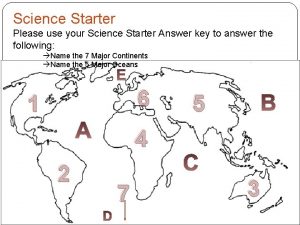
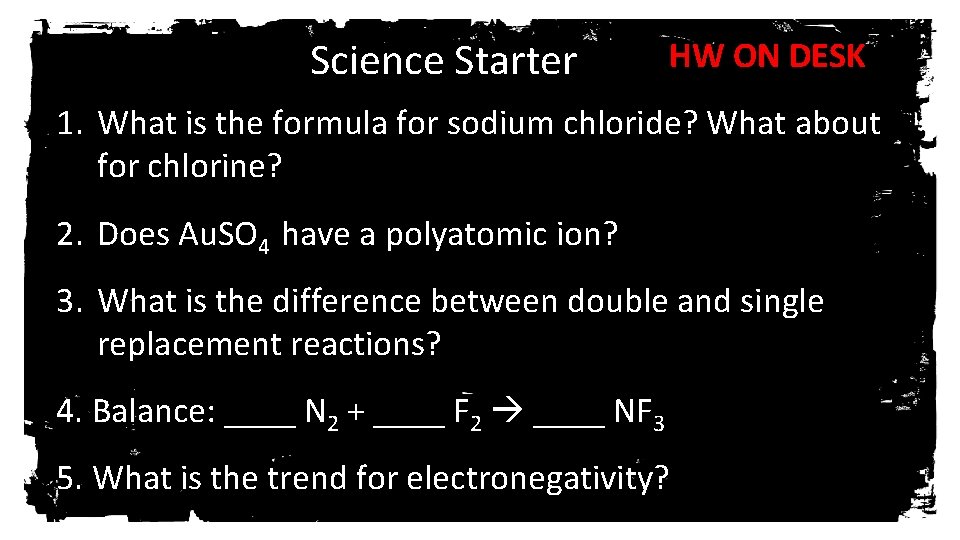
Science Starter HW ON DESK 1. What is the formula for sodium chloride? What about for chlorine? 2. Does Au. SO 4 have a polyatomic ion? 3. What is the difference between double and single replacement reactions? 4. Balance: ____ N 2 + ____ F 2 ____ NF 3 5. What is the trend for electronegativity?

Objective • SWBAT predict the products for both single and double replacement reactions with balanced equations • SWBAT read and understand the activity series

Agenda • Science Starter • Predicting Products – Single Replacement – Double Replacement – Practice • Exit Ticket/Homework

Unit 4: Chemical Reactions Predicting Products

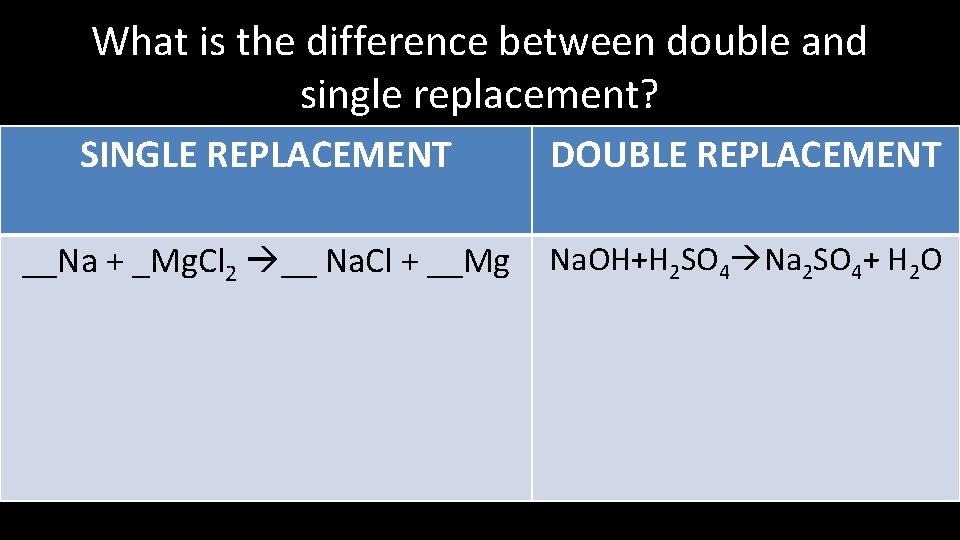
What is the difference between double and single replacement? SINGLE REPLACEMENT DOUBLE REPLACEMENT __Na + _Mg. Cl 2 __ Na. Cl + __Mg Na. OH+H 2 SO 4 Na 2 SO 4+ H 2 O

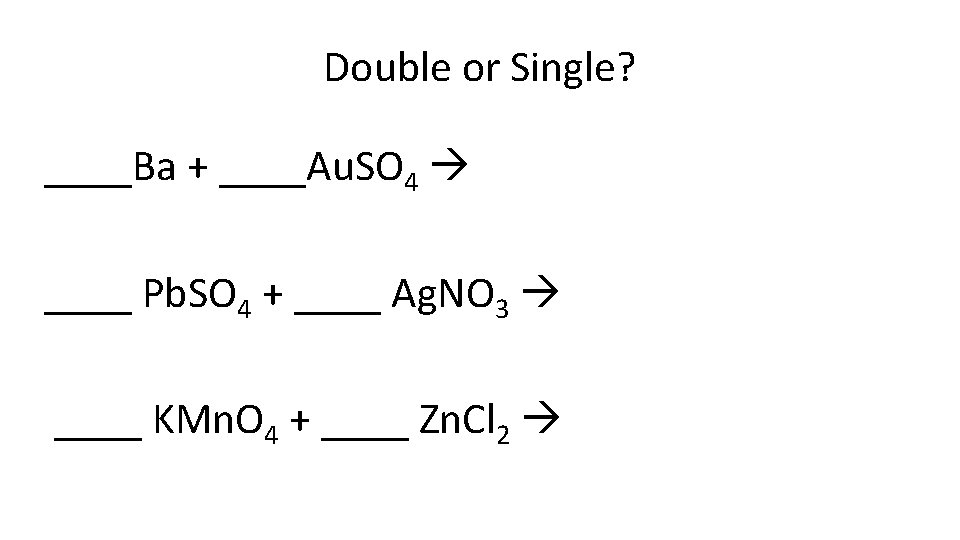
Double or Single? ____Ba + ____Au. SO 4 ____ Pb. SO 4 + ____ Ag. NO 3 ____ KMn. O 4 + ____ Zn. Cl 2

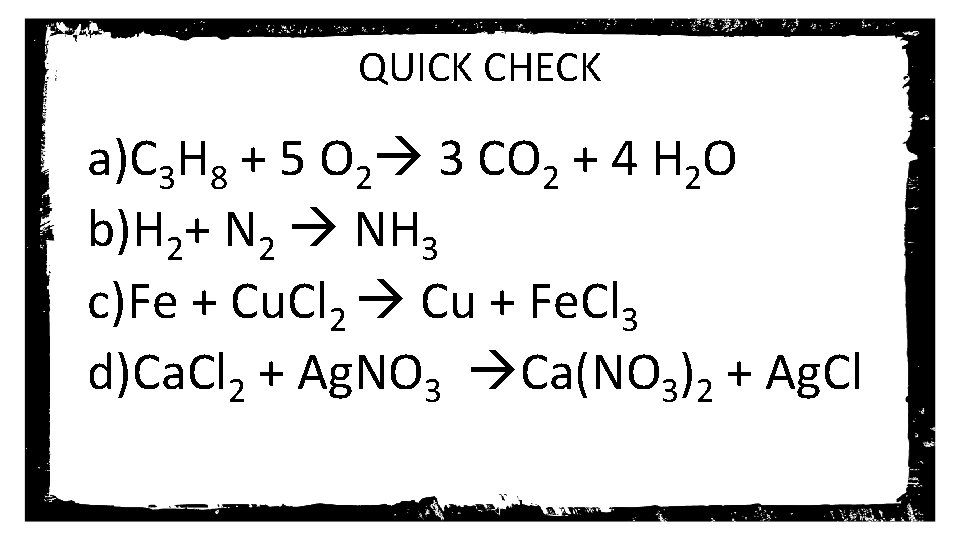
QUICK CHECK a)C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O b)H 2+ N 2 NH 3 c)Fe + Cu. Cl 2 Cu + Fe. Cl 3 d)Ca. Cl 2 + Ag. NO 3 Ca(NO 3)2 + Ag. Cl

Notes Predicting Products – Single Replacements Definition: One element replaces another element A +BC AC +B

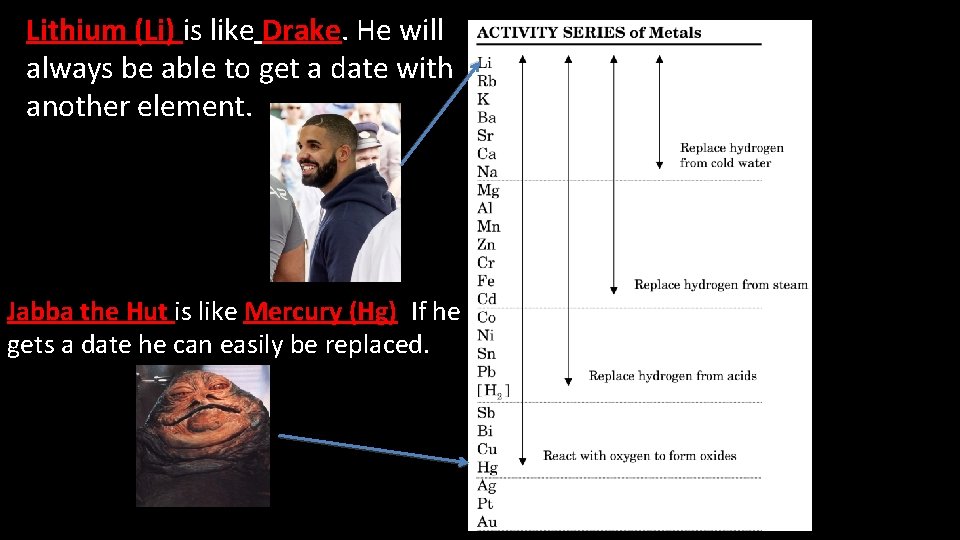
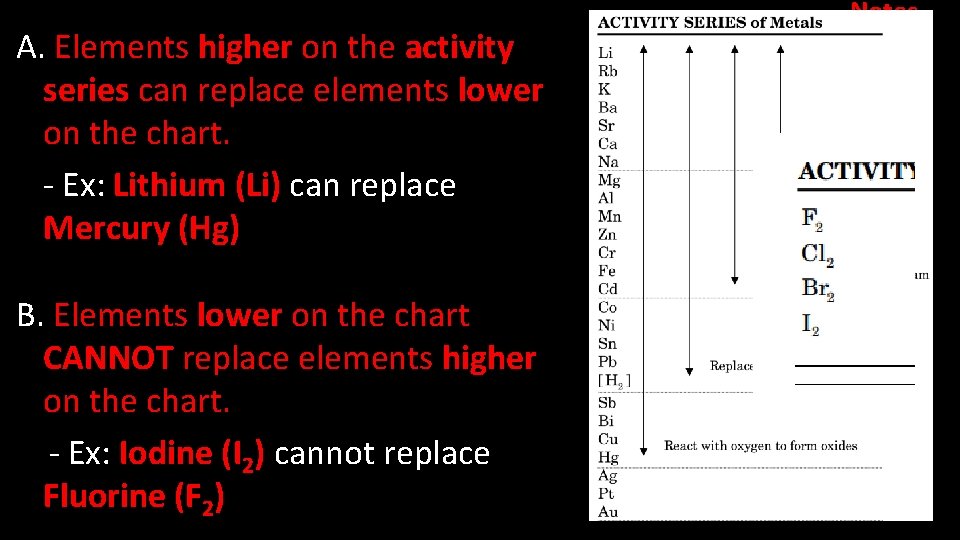
Who would have an easier time finding a date? Or If you were dating Jabba the Hut, would you replace it with Drake?

Lithium (Li) is like Drake. He will always be able to get a date with another element. Jabba the Hut is like Mercury (Hg). If he gets a date he can easily be replaced.


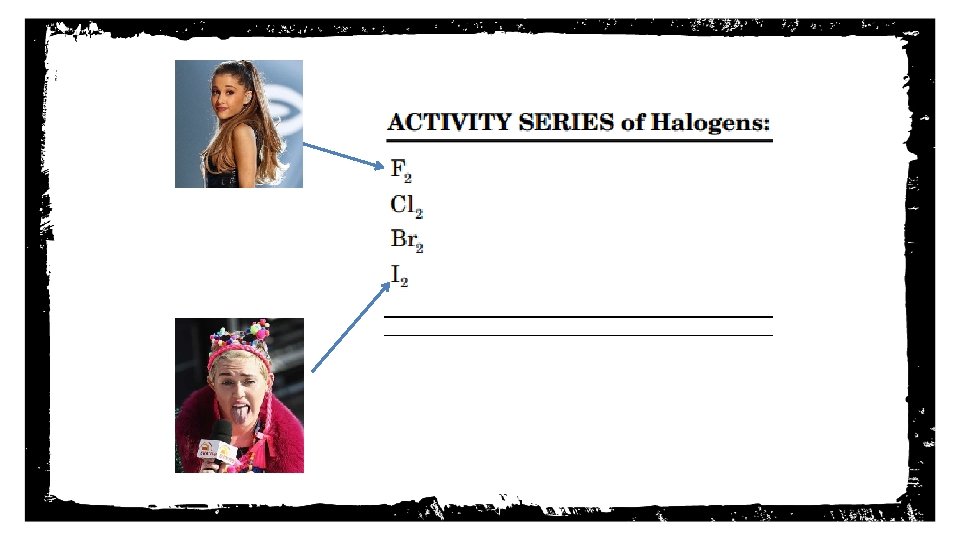
A. Elements higher on the activity series can replace elements lower on the chart. - Ex: Lithium (Li) can replace Mercury (Hg) B. Elements lower on the chart CANNOT replace elements higher on the chart. - Ex: Iodine (I 2) cannot replace Fluorine (F 2) Notes

C. Could this reaction occur? Example Problem: K + Ba. Cl 2 Ba + KCl Why? Notes

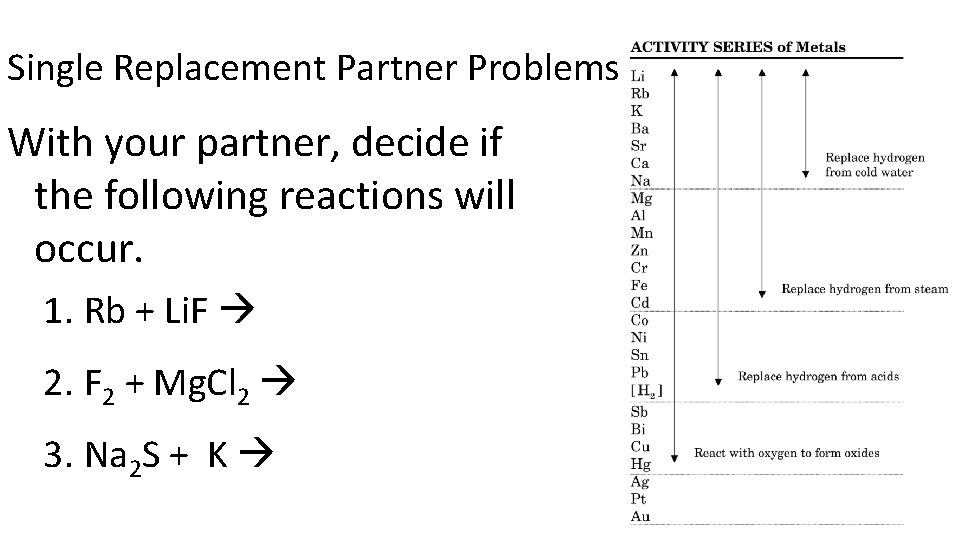
Single Replacement Partner Problems With your partner, decide if the following reactions will occur. 1. Rb + Li. F 2. F 2 + Mg. Cl 2 3. Na 2 S + K

Single Replacement – Predicting the Products • Predict the products of the following reactions: Ex. KF + Li STEPS: 1. Determine which are switching (metals or halogens? ) 2. Will partnered element trade up? (Look at activity series) 3. If the reaction will occur, swap, drop, and chop and balance equation

You Try! Predict the products of the following reactions: 1. Na. Cl + F 2 2. Li 2 O + Na

Predicting Products – Double Replacement

Notes Predicting Products – Double Replacement: Elements switch partners AB + CD AD + BC

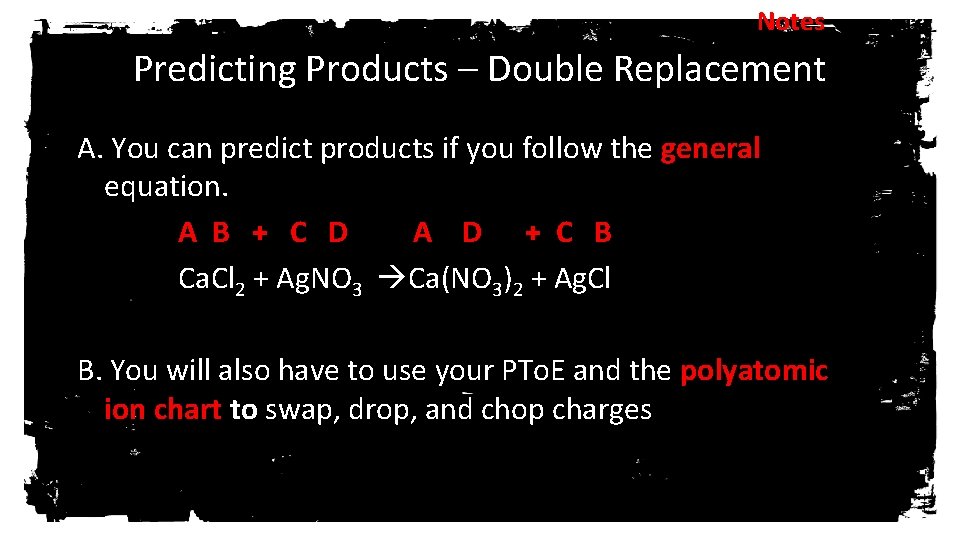
Notes Predicting Products – Double Replacement A. You can predict products if you follow the general equation. A B + C D A D + C B Ca. Cl 2 + Ag. NO 3 Ca(NO 3)2 + Ag. Cl B. You will also have to use your PTo. E and the polyatomic ion chart to swap, drop, and chop charges

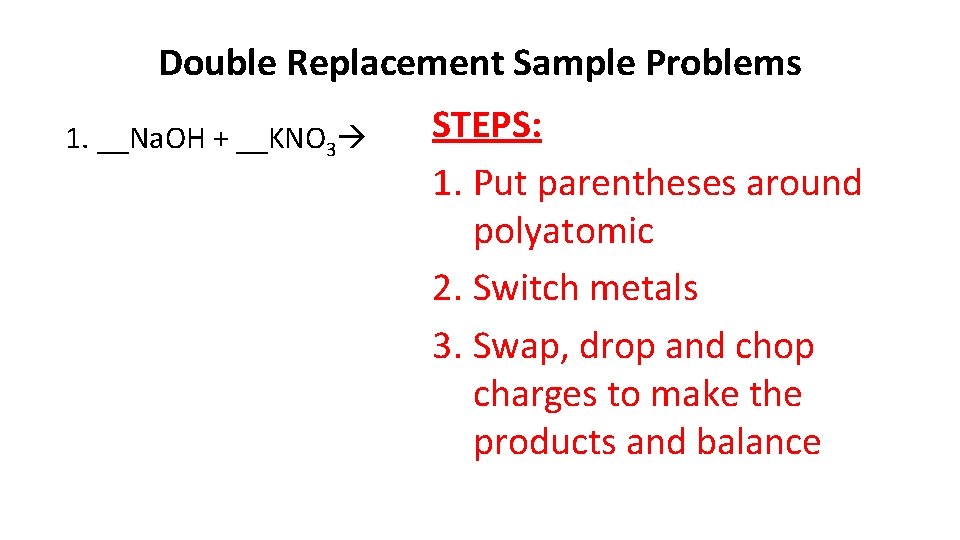
Double Replacement Sample Problems 1. __Na. OH + __KNO 3 STEPS: 1. Put parentheses around polyatomic 2. Switch metals 3. Swap, drop and chop charges to make the products and balance

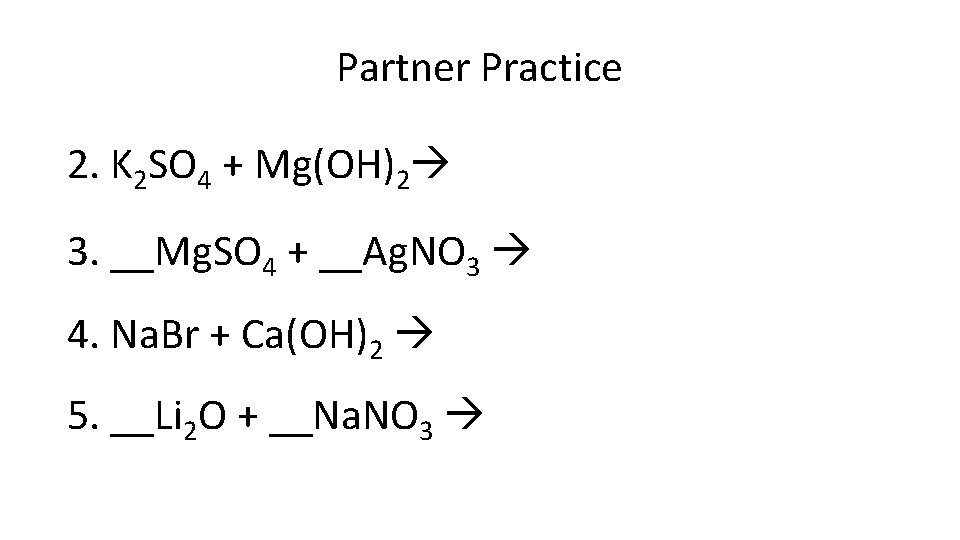
Partner Practice 2. K 2 SO 4 + Mg(OH)2 3. __Mg. SO 4 + __Ag. NO 3 4. Na. Br + Ca(OH)2 5. __Li 2 O + __Na. NO 3

Group Work Take 2 • You should not be moving around the classroom. • I should not see any cell phones. • I should not hear your voice over anyone else’s. • You should be ON TASK. • If you have any questions, ask 3 before you ask me.

PRE-LAB WORK • You must work with your group to complete “Part II” of your Homework packet… it will help you in turning verbal equations into formula equations!! You will need to do this in your lab report/write-up tomorrow! (aq) = aqueous (g) = gas (l) = liquid (s) = solid

Exit Ticket 1. Consider this incomplete chemical equation: Ba + Cu. Cl 2 What are the products of this equation? A. Ba. Cl 2 and Cu. Cl 2 B. Ba. Cu. Cl 2 and Ba C. Ba. Cl 2 and Cu D. Ba. Cu and Cl 2 BIG QUIZ TOMORROW!! WORKSHEET #4 is HOMEWORK!
 What is your favorite science subject
What is your favorite science subject Starters, movers, flyers ket pet
Starters, movers, flyers ket pet Pictogram starter
Pictogram starter Reichstag fire who was the fire starter
Reichstag fire who was the fire starter Unscramble starter
Unscramble starter Directional hypothesis example
Directional hypothesis example Starter background
Starter background Abiotic factors clipart
Abiotic factors clipart Starter activity
Starter activity Solving equations starter
Solving equations starter Rounding starter
Rounding starter Romeo and juliet starter
Romeo and juliet starter Column starter bars
Column starter bars Ransum babi
Ransum babi Novelle kjennetegn
Novelle kjennetegn Starter which muscles do you already know
Starter which muscles do you already know Midlife crisis starter pack
Midlife crisis starter pack Biological ennoblement
Biological ennoblement Location of fold mountains
Location of fold mountains Starter wordle words
Starter wordle words Nth term starter
Nth term starter Good morning starter
Good morning starter Starter
Starter Starter s11
Starter s11 Multiply starter
Multiply starter