Science Fair Project Evaporation of Liquids Nikola Tesla










- Slides: 11

Science Fair Project Evaporation of Liquids Nikola Tesla Paul French James Weldon Johnson

STATEMENT OF THE PROBLEM If water, orange juice, nail polish remover, and apple juice were tested, which one would evaporate the most?

PROJECT OVERVIEW This paper reviews how different liquids can evaporate at the same or different times. It is more likely for liquids to have a unique evaporation rate, as proved in this experiment. By comparing the amount of evaporation for the substances, we might be able to predict how much they evaporate. In my experiment, I tested water, orange juice, nail polish remover, and apple juice by pouring about 500 m. L of each liquid into a plastic cup. Then, I left them into an undisturbed, dry area to make sure my results would be valid. After that, I checked the fluids for 45 minute segments, for 4 hours, while documenting the changes I observed. I then repeated the trials 3 times on all 4 liquids. My hypothesis was that the liquid with no added chemicals – in this case, water – would evaporate the most. This is because the chemical structure for each liquid differs, and the additives might create an increased evaporation time. My hypothesis was proved correct, because the water did indeed evaporate the most. Alternative reasons for this outcome is that the substances might have been disturbed, or put out a little bit longer than the others.

RESEARCH • The evaporation process is used in the manufacture of bulk drugs, particularly in the pharmaceutical industries. • Evaporation is also used in producing biological products, such as insulin. • Vitamins and water can also be purified, which improves your health. • Scientist can make medicines by producing a liquid solution of the medicine. • Scientists later evaporate the water off to produce a solid base, to which chemicals can be added.

VARIABLES • Controlled/Constant Variables: the amount of liquid, time each liquid was left out, and the temperature of each liquid • Independent Variable: the different types of liquids • Dependent Variable: the time each liquid took to evaporate • Control Variable: the water

HYPOTHESIS The hypothesis was that the liquid with no added chemicals – in this case, water – would evaporate the most.

MATERIALS ØMeasuring cup Ø 500 m. L of water Ø 500 m. L of orange juice Ø 500 m. L of nail polish remover Ø 500 m. L of apple juice ØStopwatch ØDry area Ø 12 transparent glasses

PROCEDURES 1. Get the required materials. 2. Pour 500 m. L of water into the measuring cup. 3. Then pour it into a transparent glass. 4. Repeat numbers 2 -3 on orange juice, nail polish remover, and apple juice. 5. Every 45 minutes, check to see how much water is left in the glass for each liquid, and document the changes. 6. Repeat the whole experiment 3 more times.

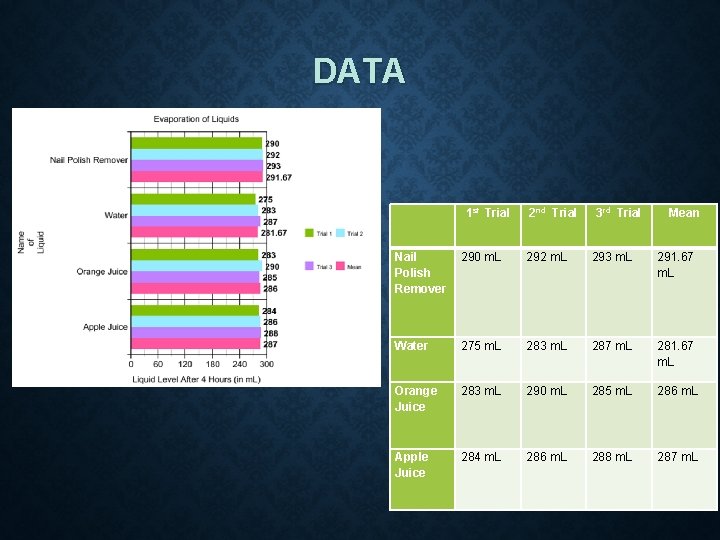
DATA 1 st Trial 2 nd Trial 3 rd Trial Mean Nail Polish Remover 290 m. L 292 m. L 293 m. L 291. 67 m. L Water 275 m. L 283 m. L 287 m. L 281. 67 m. L Orange Juice 283 m. L 290 m. L 285 m. L 286 m. L Apple Juice 284 m. L 286 m. L 288 m. L 287 m. L

CONCLUSION The researcher's hypothesis was that the purer the substance, the more it will evaporate. In this experiment, the liquid that evaporated the most was water, with a mean value of 281. 67 m. L. The second highest was orange juice, with a mean value of 286 m. L, or an average of 4. 33 m. L less than the water. Third highest was the apple juice, with a mean value of 287 m. L, or an average of 1 m. L less than the orange juice. Finally, the liquid that evaporated the least was nail polish remover, with a mean value of 291. 67 m. L, or an average of 4. 67 m. L less than the apple juice. According to the United States Geological Survey (USGS), the purest substance was water, then followed by the juices and nail polish remover. These results indicate that the purer the substance, the more it will evaporate. Thus, the researcher’s hypothesis was supported.

BIBLIOGRAPHY • Society for Science and the Public. 2015 International Rules for Pre-College Research. Sept. 28, 2015. http: //sciserv. org/isef/about/rules. • education. com. “Do All Liquids Evaporate At the Same Rate? ” | Science Project. | Education. com, 10 April, 2012, www. education. com/sciencefair/article/do-all-liquids-evaporate-at-the-same-time/. • Studios, Andrew Rader. “Evaporation of Liquids. ” Chemistry Basics, 1997, www. chem 4 kids. com/files/matter_evap. html. • Sampaolo, Mark. “Evaporation. ” Encyclopedia Britannica, Inc. , 20 Sept. 2016, www. britannica. com/science/evaporation. • Periman, Howard. “The Water Cycle: Evaporation, The Water Cycle, 2 Dec. 2016, https: //water. usgs. gov/edu/watercycleevaporation. html