Science 9 YEAR END PAT REVIEW Unit A










- Slides: 27

Science 9 YEAR END PAT REVIEW

Unit A: Biological Diversity � Biological Diversity – what is it? Why is it important? Where is the most? � Sexual Reproduction vs. Asexual Reproduction � Mitosis vs. Meiosis � Heritable vs. Non-heritable Characteristics � Discrete vs. Continuous variation

Biological Diversity – what is it? Why is it important? Where is the most? � Biological Diversity – refers to all the different types of organisms on Earth ◦ Diversity within an ecosystem ◦ Diversity with a species � Species Distribution ◦ Areas around the equator have the greatest number of plant and animal species. Why? � Why is diversity good? ?

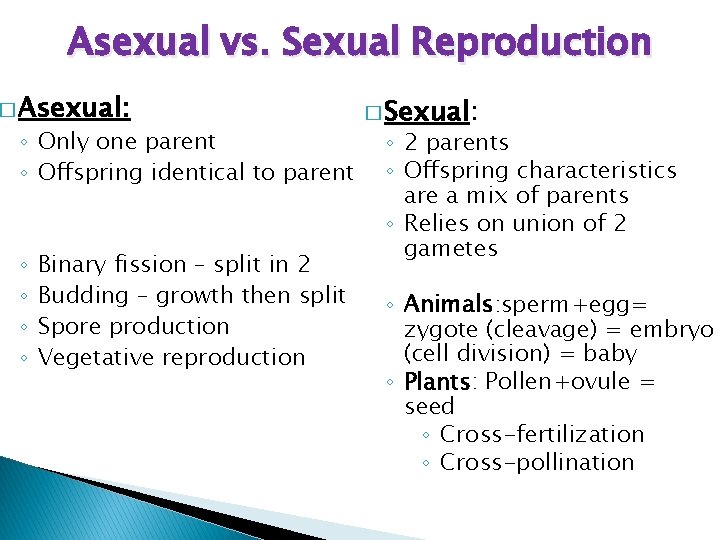
Asexual vs. Sexual Reproduction � Asexual: ◦ Only one parent ◦ Offspring identical to parent ◦ ◦ Binary fission – split in 2 Budding – growth then split Spore production Vegetative reproduction � Sexual: ◦ 2 parents ◦ Offspring characteristics are a mix of parents ◦ Relies on union of 2 gametes ◦ Animals: sperm+egg= zygote (cleavage) = embryo (cell division) = baby ◦ Plants: Pollen+ovule = seed ◦ Cross-fertilization ◦ Cross-pollination

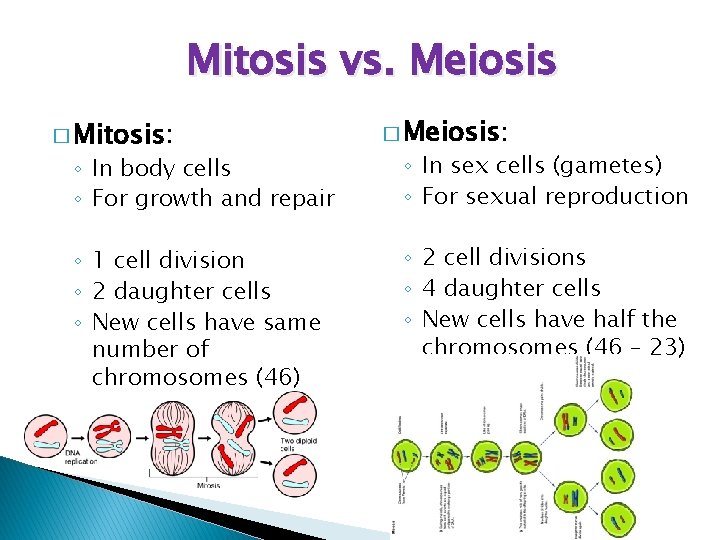
Mitosis vs. Meiosis � Mitosis: ◦ In body cells ◦ For growth and repair ◦ 1 cell division ◦ 2 daughter cells ◦ New cells have same number of chromosomes (46) � Meiosis: ◦ In sex cells (gametes) ◦ For sexual reproduction ◦ 2 cell divisions ◦ 4 daughter cells ◦ New cells have half the chromosomes (46 – 23)

Heritable vs. Non-heritable Characteristics � Heritable: ◦ Passed on from generation to generation ◦ Ex: eye color, hair type, hair color � Non-heritable: ◦ Are acquired. ◦ Not passed on from generation to generation ◦ Ex: learning to play the piano, dyed hair

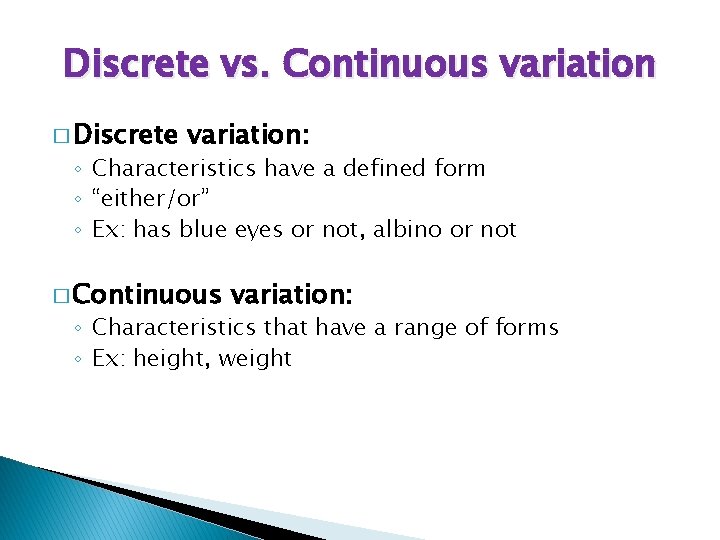
Discrete vs. Continuous variation � Discrete variation: ◦ Characteristics have a defined form ◦ “either/or” ◦ Ex: has blue eyes or not, albino or not � Continuous variation: ◦ Characteristics that have a range of forms ◦ Ex: height, weight

Unit B: Matter and Chemical Change � Metal vs. Non-metal – where to find on periodic table? Common properties. � Chemical vs. Physical change � # of protons/electrons/neutrons � Naming compounds - # of elements, # of atoms � Ionic vs. Molecular compounds � Law of conservation of mass

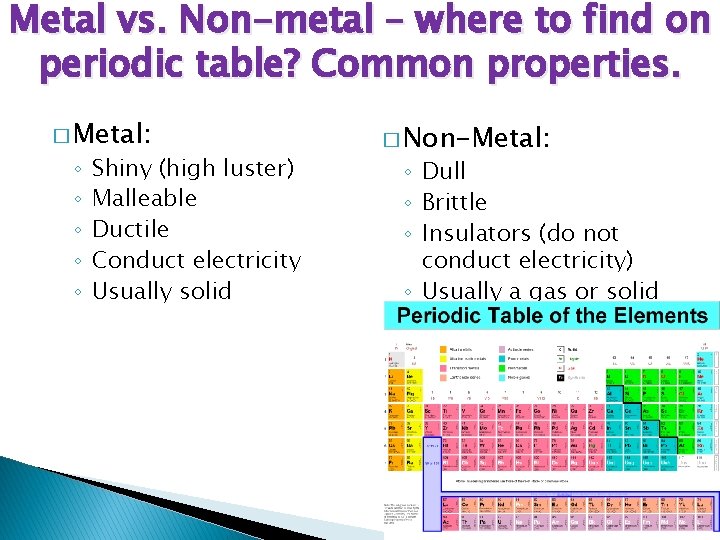
Metal vs. Non-metal – where to find on periodic table? Common properties. � Metal: ◦ ◦ ◦ Shiny (high luster) Malleable Ductile Conduct electricity Usually solid � Non-Metal: ◦ Dull ◦ Brittle ◦ Insulators (do not conduct electricity) ◦ Usually a gas or solid

Chemical vs. Physical change � Physical Change – appearance or state may change, but composition does not. ◦ Ex: melting, freezing � Chemical Change – always results in the formation of a new substance ◦ Ex: cooking, fire � Evidence: 1. 2. 3. 4. Change in color Change in odor Formation of a solid or gas Release or absorption of heat

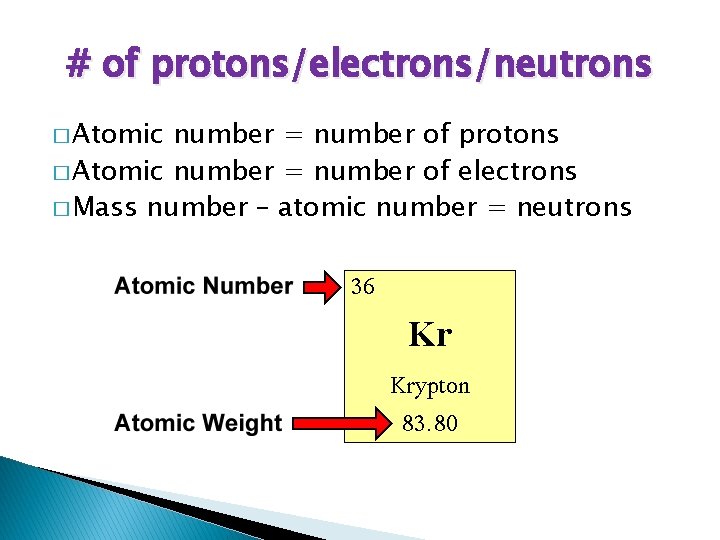
# of protons/electrons/neutrons � Atomic number = number of protons � Atomic number = number of electrons � Mass number – atomic number = neutrons

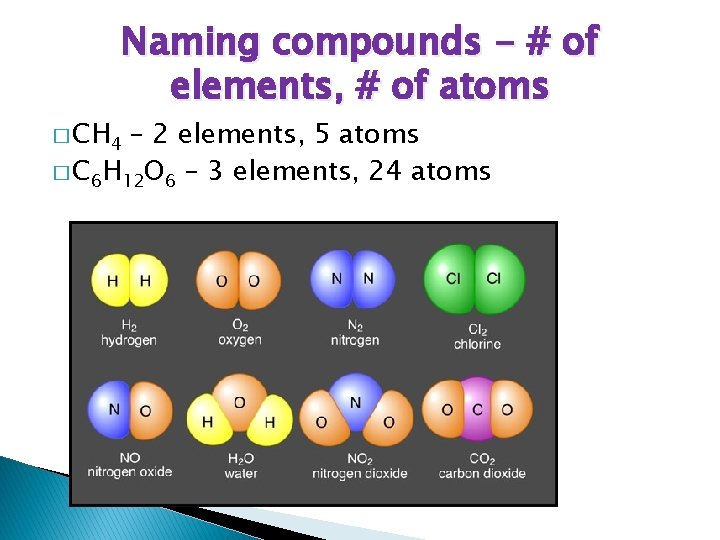
Naming compounds - # of elements, # of atoms � CH 4 – 2 elements, 5 atoms � C 6 H 12 O 6 – 3 elements, 24 atoms

Ionic vs. Molecular compounds � Ionic Compounds One metal, one non-metal Formed from the attraction of opposite charges Always solid at room temp Conduct electricity Naming: use ions. Switch ion charges with number of atoms. (use roman numerals when more than one charge. ◦ Ex: Na. Cl ◦ ◦ ◦ � Molecular ◦ ◦ ◦ Compounds 2 non-metals Can be solid, liquid or gas Insulators Low melting and boiling points Naming: use prefixes (mono, di, tri, tetra, penta, etc) Ex: Sugar, water

Law of conservation of mass � Matter is neither created or destroyed in a chemical reaction � Mass before reaction = mass after reaction ◦ In a closed system � If not closed, some matter can escape. Usually a gas.

Unit C: Environmental Chemistry � Organic � LD 50 � Effects vs. Inorganic of Nitrogen in a lake � p. H – acid vs. Base – properties

Organic vs. Inorganic � Organic ◦ ◦ compounds contain Carbohydrates Lipids (fats) Protein Nucleic Acids

LD 50 � The concentration, or amount of a substance that would kill 50% of the population it is given to.

Effects of Nitrogen in a lake � More elements from fertilizers: ◦ More plant life, less fish can live in this ◦ Plants start to die ◦ Bacteria use dead plant as food and decompose the plants ◦ With more food, more bacteria populate ◦ All this bacteria need oxygen to survive, so they use up much of the dissolved oxygen in the water ◦ The lake is no longer a place where fish and many insects can live. ◦ See p. 219

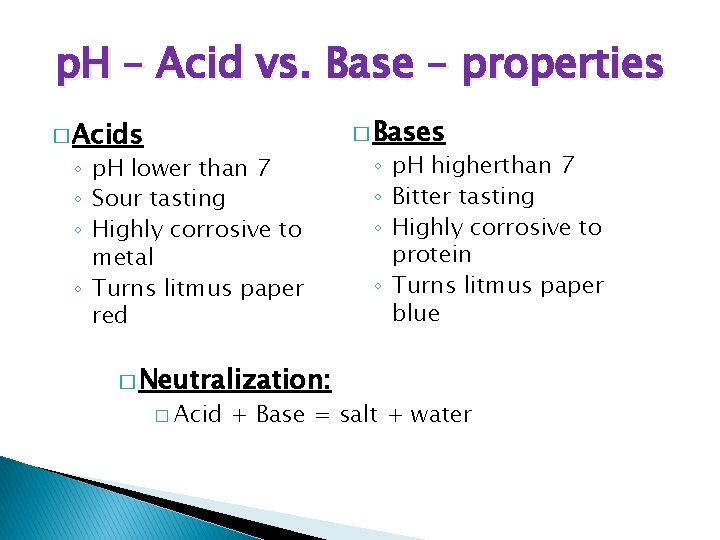
p. H – Acid vs. Base – properties � Acids ◦ p. H lower than 7 ◦ Sour tasting ◦ Highly corrosive to metal ◦ Turns litmus paper red � Neutralization: � Acid � Bases ◦ p. H higherthan 7 ◦ Bitter tasting ◦ Highly corrosive to protein ◦ Turns litmus paper blue + Base = salt + water

Unit D: Principles of Electricity � static vs. current electricity � electromagnets � series vs. parallel circuits � efficiency � wet vs. dry cells � resistors

Static vs. Current electricity � Static – a build up of charges causing attraction. Stationary electrons � Current – electrons moving along a path ◦ Useful form ◦ Requires and energy source, a path, and a load

Electromagnets � Wire wrapped around iron, when current passes through, a magnet is produced � Used in motors � Wire wrapped around an iron core � The more wraps, the stronger the magnet.

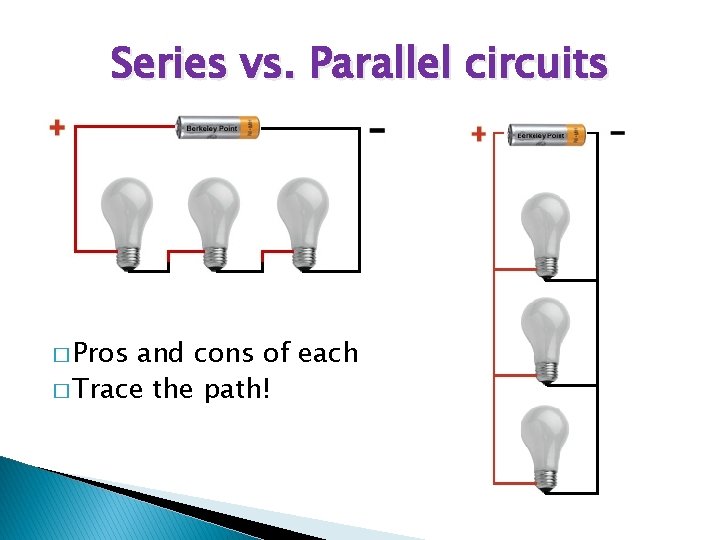
Series vs. Parallel circuits � Pros and cons of each � Trace the path!

Efficiency � Almost all devices lose energy ◦ Usually to heat, light, sound etc. � To calculate out efficiency: � Useful output Input energy x 100 = % efficiency

Wet vs. Dry cells � Wet cells � Dry cells ◦ ◦ ◦ ◦ First cells made Cheep to make Liquid Electrolyte (usually a strong acid) 2 Metal Electrodes (usually copper and zinc) Expensive Paste electrolyte 2 metal electrodes Convenient, can be in any position and not spill

Resistors � Resistors electrons – slow down the movement of ◦ Slow them down so much that it can cause heat and light ◦ This is how regular incandescent light bulbs work, they have a tungsten filament that is a resistor ◦ Toaster has a resistor to heat it up � Variable Resistor (rheostat) – control the amount of resistance. Ex: Light dimmer, volume control.

Unit E: Space Exploration � inner vs. outer planets � azimuth and altitude � spectroscope � red/blue shift – Doppler effect � reflective and refractive telescopes