Science 9 Unit 1 Chemistry Lesson 1 Matters










- Slides: 15

Science 9 Unit 1: Chemistry Lesson 1: Matters

Objectives By the end of the lesson you should be able to: �Describe matter using physical properties �Use the KMT to describe changes of state �Explain the difference between an element and a compound

Matter �Matter volume is anything that has mass and ◦ Mass: amount of matter in a substance (g) ◦ Volume: amount of space a substance occupies (L) �Everything around us is matter!

Changes in Matter �Matter cannot be created nor destroyed �A chemical change is a change in matter than occurs when substances combined to form a new substance. ◦ HINTS: explosions, colour changes when heated �A physical change is a change in appearance but no new substance is formed ◦ HINTS: state changes

Describing Matter On blue paper there are different ways that matter can be described With a partner separate these descriptions into 2 categories *hint look at your gold pieces of paper*

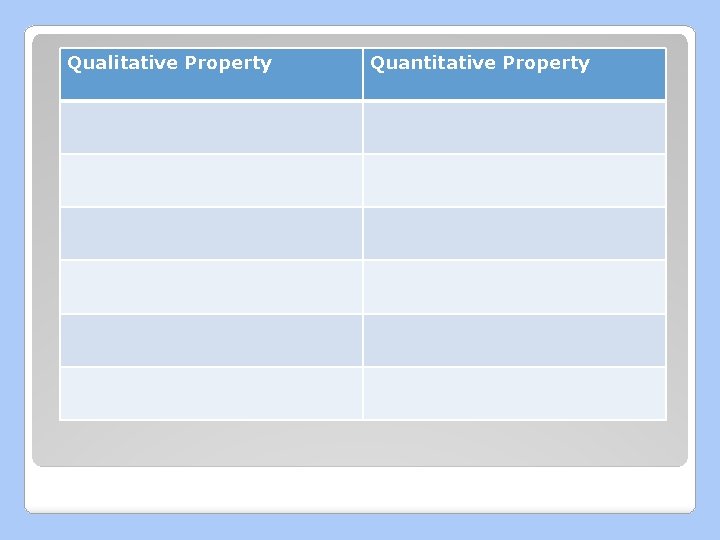
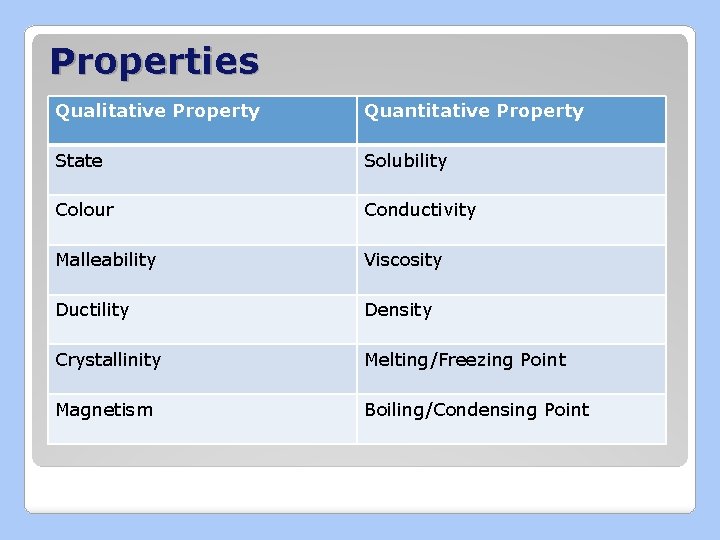
Describing Matter �Physical Properties: characteristics that can be observed or measured ◦ Qualitative: described ◦ Quantitative: measured numerically

Qualitative Property Quantitative Property

Properties Qualitative Property Quantitative Property State Solubility Colour Conductivity Malleability Viscosity Ductility Density Crystallinity Melting/Freezing Point Magnetism Boiling/Condensing Point

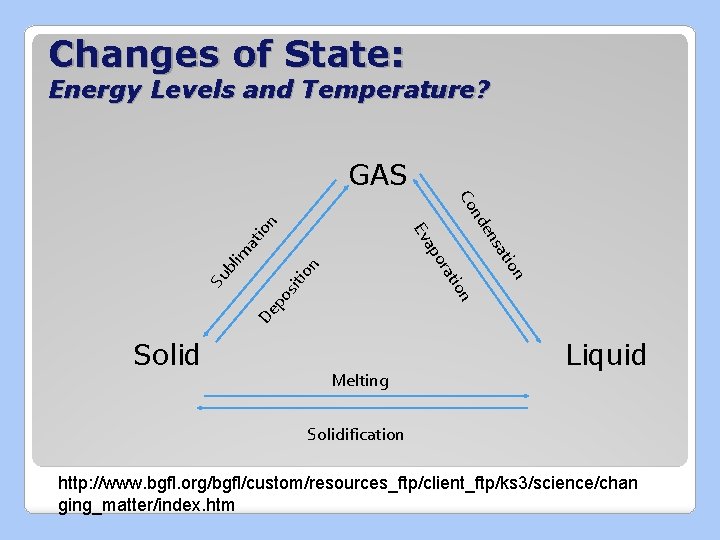
KMT: Kinetic Molecular Theory �Kinetic Energy: the energy of motion Molecular Theory: explains what happens to matter as the energy in the particles changes

KMT 1. All matter is made up of very small particles There is empty space between particles 2.

3. Particles are constantly moving a. Particles of a solid are so tightly packed together that they cannot move around freely. They can only vibrate b. Particles of a liquid are farther apart, they can move by sliding past each other c. Particles of a gas are very far apart, they move around quickly 4. Energy makes particles move. The more energy particles have, the faster they can move and the farther apart they can get

Changes of State: Energy Levels and Temperature? GAS n lim at io n io De po ion sit on ati ns t ora ap Su b e nd Co Ev Solid Melting Liquid Solidification http: //www. bgfl. org/bgfl/custom/resources_ftp/client_ftp/ks 3/science/chan ging_matter/index. htm

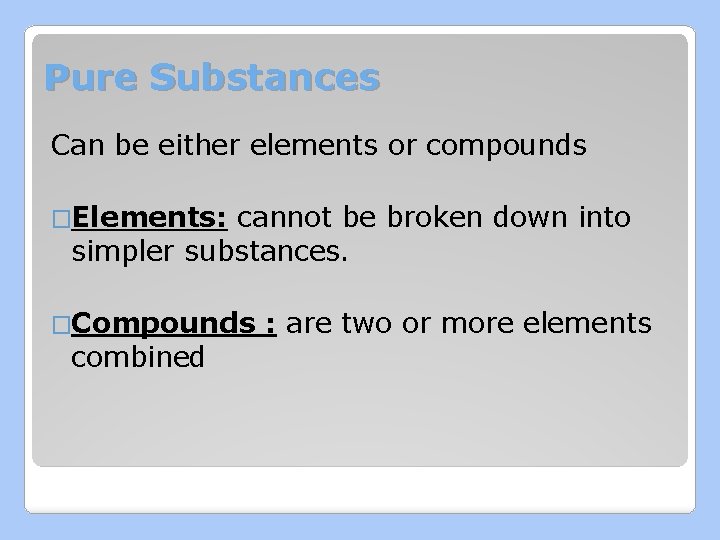
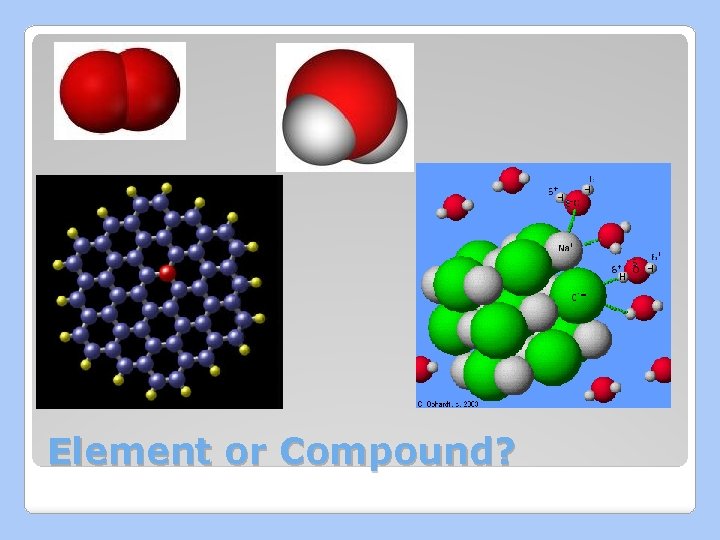
Pure Substances Can be either elements or compounds �Elements: cannot be broken down into simpler substances. �Compounds combined : are two or more elements

Element or Compound?

Your Turn! �Activity Time: Describing Matter Lab!