Science 9 Chem 6 Naming Ionic Compounds Objectives
Science 9 Chem 6: Naming Ionic Compounds

Objectives By the end of the lesson you should be able to: ¡ Describe simple and complex ions ¡ Name and make all types of ionic compounds
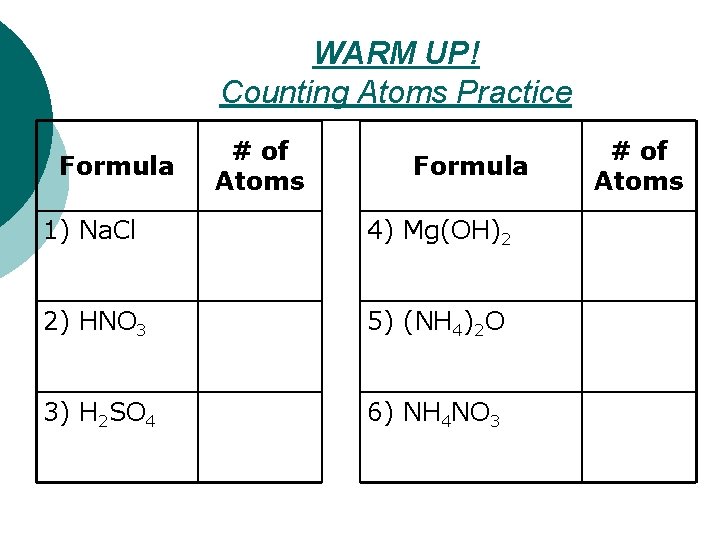
WARM UP! Counting Atoms Practice Formula # of Atoms Formula 1) Na. Cl 4) Mg(OH)2 2) HNO 3 5) (NH 4)2 O 3) H 2 SO 4 6) NH 4 NO 3 # of Atoms
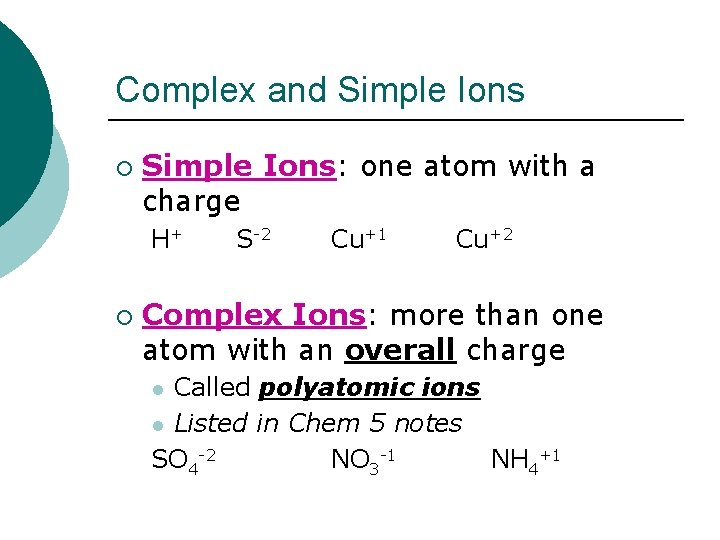
Complex and Simple Ions ¡ Simple Ions: one atom with a charge H+ ¡ S-2 Cu+1 Cu+2 Complex Ions: more than one atom with an overall charge Called polyatomic ions l Listed in Chem 5 notes SO 4 -2 NO 3 -1 NH 4+1 l

Remember… ¡ ALL ELEMENTS WANT TO HAVE FULL OUTER SHELLS! The combining capacity (AKA: charge) tells you how many electrons have been lost/gained to get a full outer shell
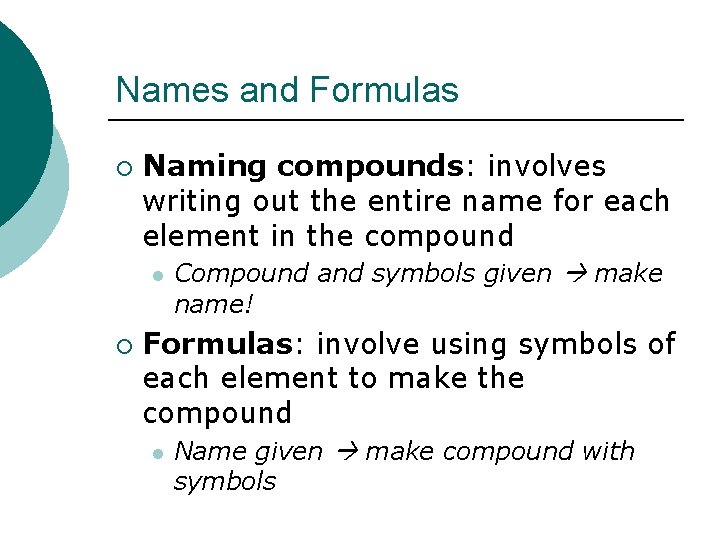
Names and Formulas ¡ Naming compounds: involves writing out the entire name for each element in the compound l ¡ Compound and symbols given make name! Formulas: involve using symbols of each element to make the compound l Name given make compound with symbols

Remember Ionic Compounds…. Always involve a positive charge (metal) and a negative charge (non-metal) ¡ Electrons are always transferred from the positive to the negative ¡ Create ions because losing and gaining electrons (charges result) ¡

Naming Ionic Compounds Metal always first; name never changes ¡ Non-metal second; ending becomes “ide” ¡ EX: Na. Cl = sodium chloride Mg. F 2 = magnesium fluoride Al 2 O 3 = aluminum oxide

Your Turn! ¡ Practice Problems
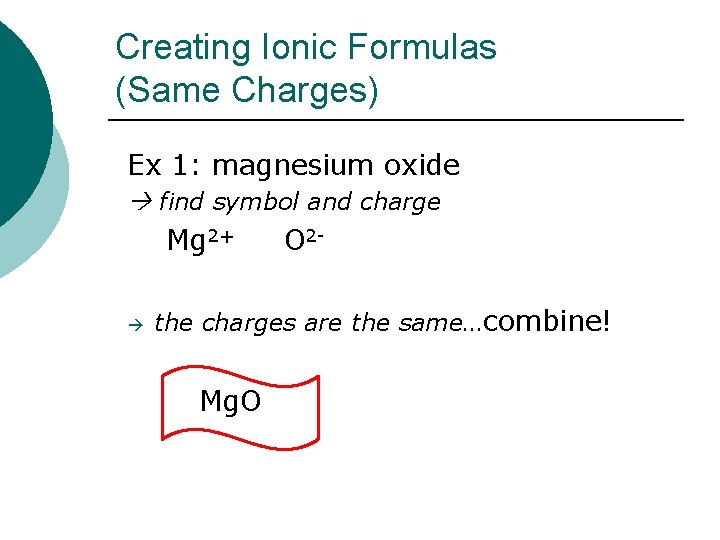
Creating Ionic Formulas (Same Charges) Ex 1: magnesium oxide find symbol and charge Mg 2+ O 2 - the charges are the same…combine! Mg. O
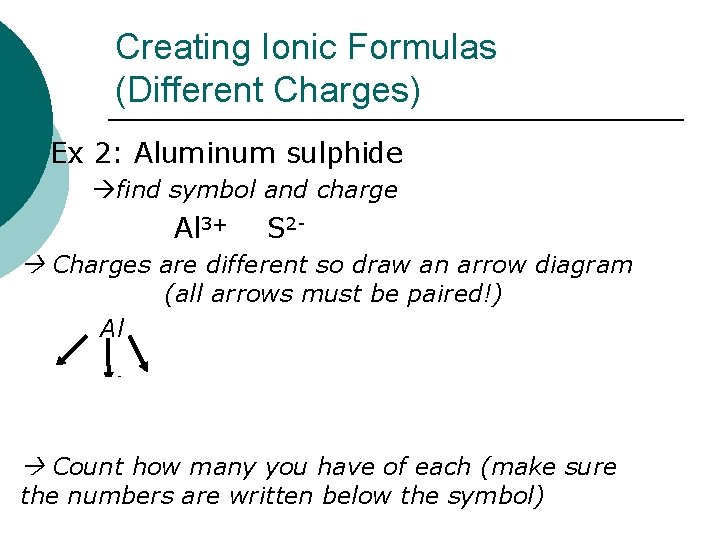
Creating Ionic Formulas (Different Charges) Ex 2: Aluminum sulphide find symbol and charge Al 3+ S 2 - Charges are different so draw an arrow diagram Al (all arrows must be paired!) Al Al 2 S 3 S S S Count how many you have of each (make sure the numbers are written below the symbol)

Your Turn! ¡ Practice Problems
- Slides: 12