SCIENCE 3 Week One LESSON 1 PROPERTIES OF





















- Slides: 28

SCIENCE 3 Week One

LESSON 1 : PROPERTIES OF MATTER

What I Need To Know Describe different objects based on their characteristics such as shape, weight, volume, ease of flow, and others Tell that matter occupies space and has mass

What I Know • Look around you. Name some objects around as you recite the poem



Matter Is All Around Here, there, and everywhere, all You can see is matter. It may be red or blue, Small and bouncy as a ball. Or large and heavy As a house or a bull.

It may be a round, a square Or a rectangular table. Just like flowing water in a river or leaking faucet or the fluffy white clouds

in the sky during the day truly matter is all around Including you and me.

What’s In All that you can see around you is matter. Plants, animals, and even the smallest or largest object around you is matter. All kinds of matter have distinguishing characteristics

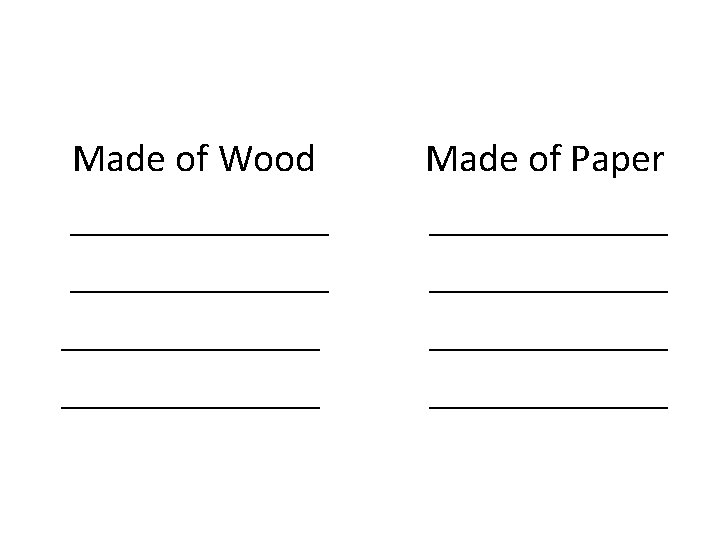
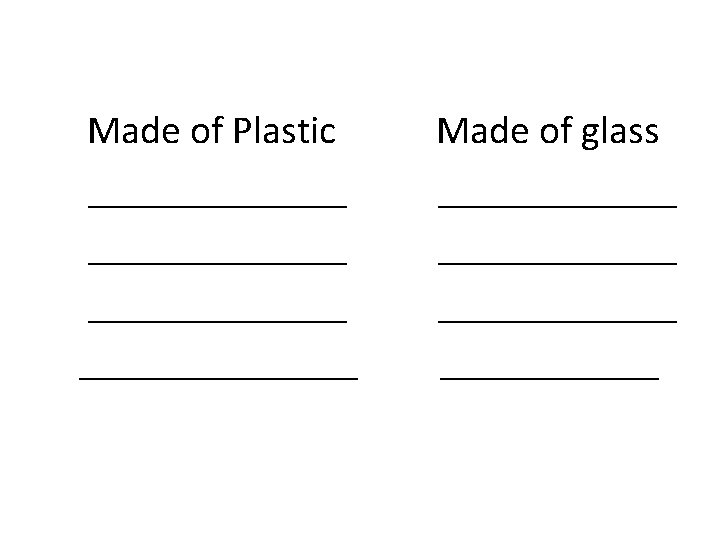
Knowing these properties help you deal and utilize matter properly. Direction: Make a list of the things around you. Group the things according to their characteristics.

Made of Wood _____________ Made of Paper ____________

Made of Plastic ______________ Made of glass ____________ ______

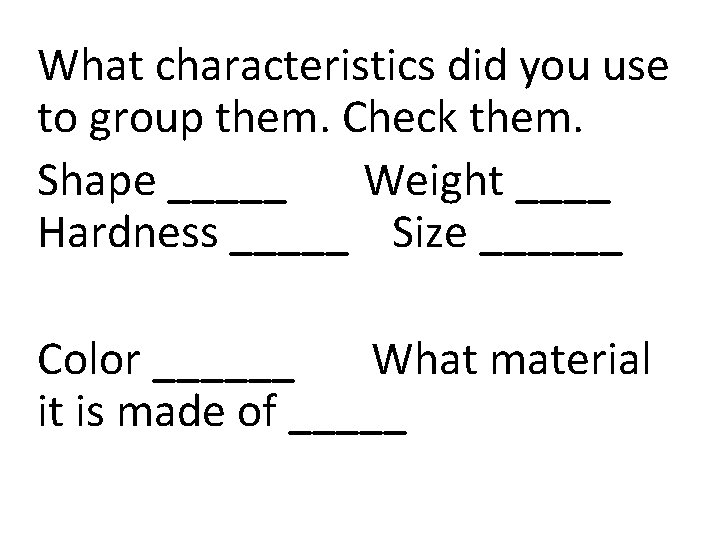
What characteristics did you use to group them. Check them. Shape _____ Weight ____ Hardness _____ Size ______ Color ______ What material it is made of _____

What’s New Anything we see or do not see is matter. Matter is a Why does matter comes in different sizes and shapes ? Well that is because matter comes in three forms; solid, liquid and gas. Anything which occupies space and has mass.

Why does matter comes in different sizes and shapes ? Well that is because matter comes in three forms; solid, liquid and gas. Let us explore more about them and their properties

What Is It Physical properties are determined without changing the identity of the substance. Physical properties of matter can be classified as intensive and extensive

Intensive – Properties that do not depend on the amount of matter present. Color – shade, tint, or pigment of materials Odor – pleasant or unpleasant scent of materials Luster – luminous or shiny Malleability – the ability of a material to be beaten into thin sheets without breaking. Ductility – the abilty of a material to allow the flow of energy or electricity Hardness – resistance of a solid material from being scratch

Extensive – Properties depend on the amount of matter present, such as; Mass – the amount of matter in an object; it is expressed in grams; we use a weighing scale or a platform to find the mass of an object Weight - the gravitational force of Earth acting on an object Volume – the amount of space that an object occupies

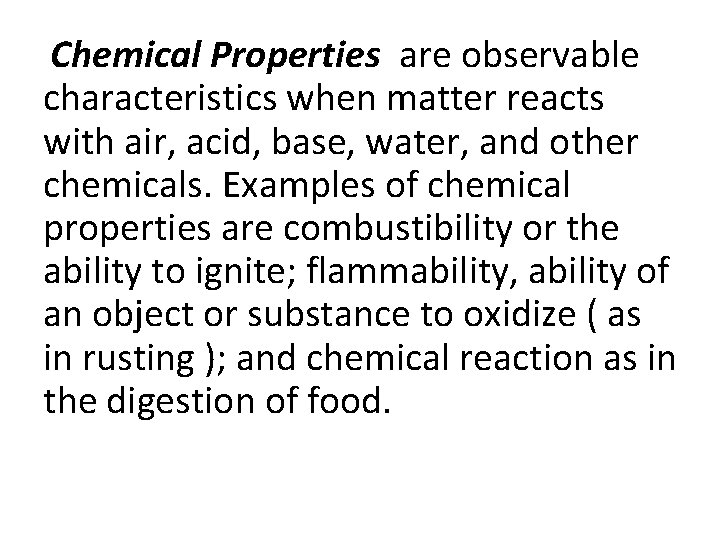
Chemical Properties are observable characteristics when matter reacts with air, acid, base, water, and other chemicals. Examples of chemical properties are combustibility or the ability to ignite; flammability, ability of an object or substance to oxidize ( as in rusting ); and chemical reaction as in the digestion of food.

What’s More Direction : Fill in the blanks with a word or phrase that best completes the sentence. The amount of space that a block of wood occupies is called _________. Color, odor and shape are examples of ________ property of a material perceived by body senses.

The amount of matter that makes up an object is called. The volume of the water in a drinking bottle is expressed in ______. To measure the volume of a solid object, we use ________ is the amount of space that matter occupies. Matter is anything that has mass and occupies ___________ properties are observable properties when matter reacts with air, acid, base water and other chemicals. Physical properties are determined without changing the ______ of the substance. Weight is the _______ force of Earth acting on an object.

What I Have Learned Matter is anything that has mass and occupies space. Physical properties of matter are shape, texture, color, odor, etc. Chemical properties are combustibility, flammability, rusting and digestion. •

What I Can Do Direction: Choose an object and draw it on the box. Write a paragraph to describe the characteristics of your drawing. Color it.

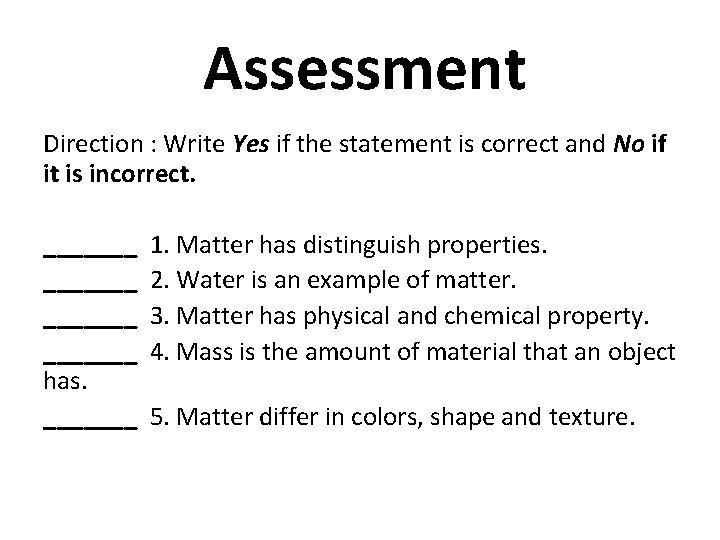
Assessment Direction : Write Yes if the statement is correct and No if it is incorrect. _______ has. _______ 1. Matter has distinguish properties. 2. Water is an example of matter. 3. Matter has physical and chemical property. 4. Mass is the amount of material that an object 5. Matter differ in colors, shape and texture.

Additional Activities Direction: Encircle the correct answer. 1. Which of the following is HOT an example of matter. a. water b. smoke c. air d. water vapor 2. Matter is defined as anything that… a. has mass and takes up space b. has energy c. has a fixed volume and weight

3. Which of the following properties can only be determind through a chemical change. a. hardness b. melting point c. mass d. size 4. A characteristics that can be observed or measured without changing the composition of a substance is ; a. chemical property b. physical property c. transitive property d. all of the above

Which of the following represents a physical change? a. Rusting b. Boiling c. cutting d. chopping