Science 10 Unit 2 Chemistry 4 2 Beautiful

Science 10 Unit 2: Chemistry 4. 2 Beautiful Bonding and Naming Compounds

Objectives By the end of the lesson you should be able to: ¡ Describe simple and complex ions ¡ Describe the differences between covalent and ionic bonds ¡ Name and make all types of ionic and covalent compounds
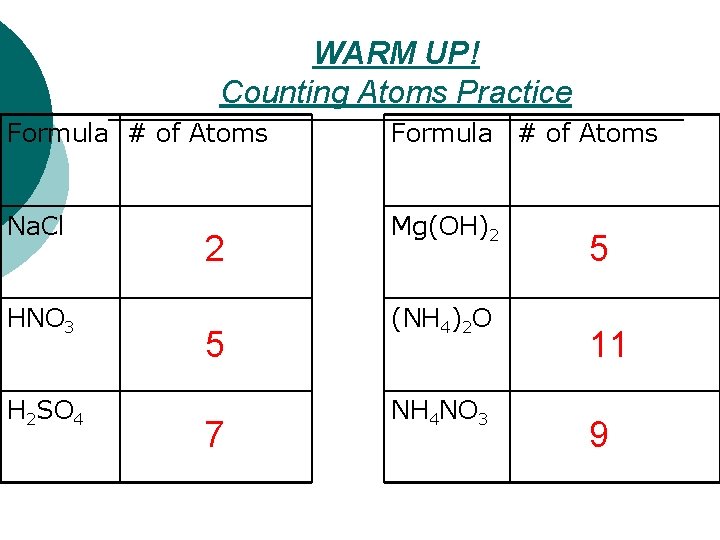
WARM UP! Counting Atoms Practice Formula # of Atoms Na. Cl Mg(OH)2 HNO 3 H 2 SO 4 2 5 7 (NH 4)2 O NH 4 NO 3 5 11 9
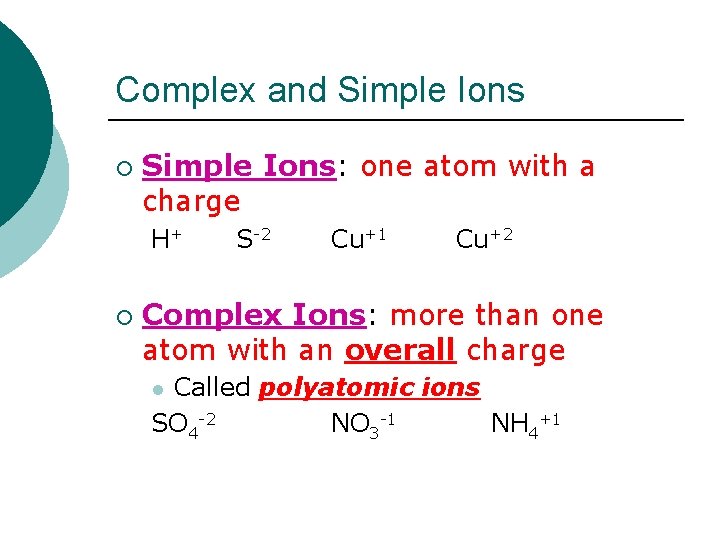
Complex and Simple Ions ¡ Simple Ions: one atom with a charge H+ ¡ S-2 Cu+1 Cu+2 Complex Ions: more than one atom with an overall charge Called polyatomic ions SO 4 -2 NO 3 -1 NH 4+1 l

Remember… ¡ ALL ELEMENTS WANT TO HAVE FULL OUTER SHELLS! The combining capacity (ion charge) tells you how many electrons have to be lost/gained to get a full outer shell

Ionic Compounds Always involve a metal and a non-metal ¡ Electrons are always transferred from the metal to the non-metal ¡ Create ions because losing and gaining electrons (charges result) ¡
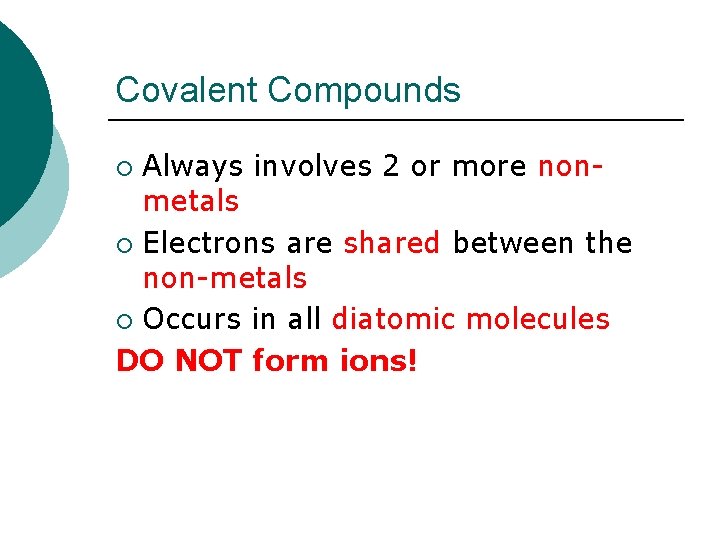
Covalent Compounds Always involves 2 or more nonmetals ¡ Electrons are shared between the non-metals ¡ Occurs in all diatomic molecules DO NOT form ions! ¡
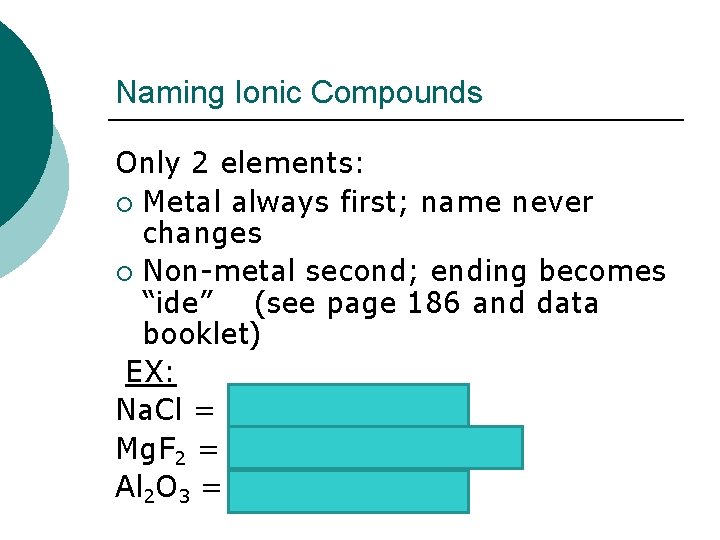
Naming Ionic Compounds Only 2 elements: ¡ Metal always first; name never changes ¡ Non-metal second; ending becomes “ide” (see page 186 and data booklet) EX: Na. Cl = sodium chloride Mg. F 2 = magnesium fluoride Al 2 O 3 = aluminum oxide

Making Ionic Compounds 1. Get symbol and charge l l Charges are the same skip to step 2 If charges are different “swap and drop” Combine Examples On Whiteboard Ex. Sodium chloride Magnesium oxide Aluminum fluoride Magnesium nitride 2.
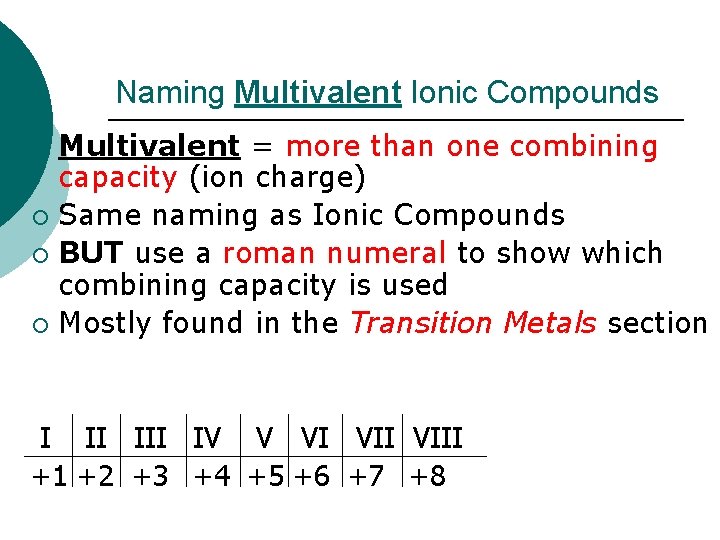
Naming Multivalent Ionic Compounds Multivalent = more than one combining capacity (ion charge) ¡ Same naming as Ionic Compounds ¡ BUT use a roman numeral to show which combining capacity is used ¡ Mostly found in the Transition Metals section ¡ I II IV V VI VIII +1 +2 +3 +4 +5 +6 +7 +8

Making Multivalent Ionic Compounds (Roman Numeral given) 1. Get appropriate ion (from Roman Numeral) l l Charges are the same skip to step 2 If charges are different “swap and drop” Combine 3. Reduce to lowest common ratio if needed. Examples on whiteboard Eg. Manganese (IV) sulfide Cobalt (III) oxide Cobalt (II) oxide 2.

Naming Multivalent Ionic Compounds (no Roman Numeral given) Write the options 2. Determine which pairing will give the correct formula 3. Write the name same as you did with Binary Ionic Compounds 4. Add the appropriate Roman Numeral between the metal and non-metal Examples on whiteboard Eg. Au 3 N Fe. O Fe 2 O 3 1.
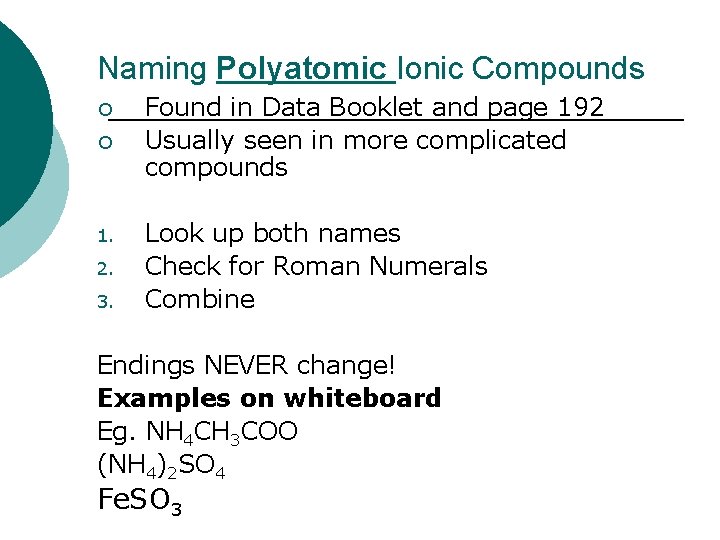
Naming Polyatomic Ionic Compounds ¡ ¡ 1. 2. 3. Found in Data Booklet and page 192 Usually seen in more complicated compounds Look up both names Check for Roman Numerals Combine Endings NEVER change! Examples on whiteboard Eg. NH 4 CH 3 COO (NH 4)2 SO 4 Fe. SO 3

Making Polyatomic Ionic Compounds 1. Get symbol and charge l l 2. If same skip to step 2 If different “swap and drop” Combine – use brackets if need be for polyatomic ions ONLY Eg. Manganese (III) chlorate Ammonium sulfate

Naming Covalent Compounds 1. 2. 3. Name first element Name second element with “ide” ending Add any needed prefixes

Prefixes Prefix Mono Di Tri Tetra Penta Hexa Hepta Octa Nona Deca # Atoms 1 2 3 4 5 6 7 8 9 10 IF first atom is 1 DO NOT add “mono” Change any “oo” to “o” I. E. monooxide = monoxide
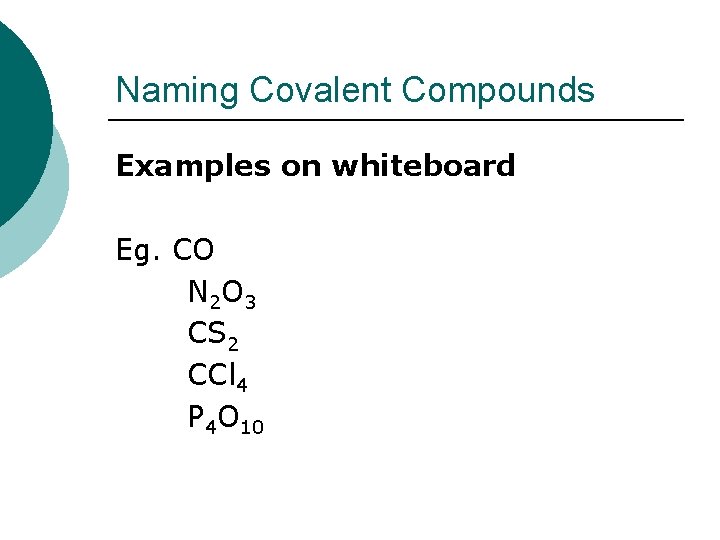
Naming Covalent Compounds Examples on whiteboard Eg. CO N 2 O 3 CS 2 CCl 4 P 4 O 10
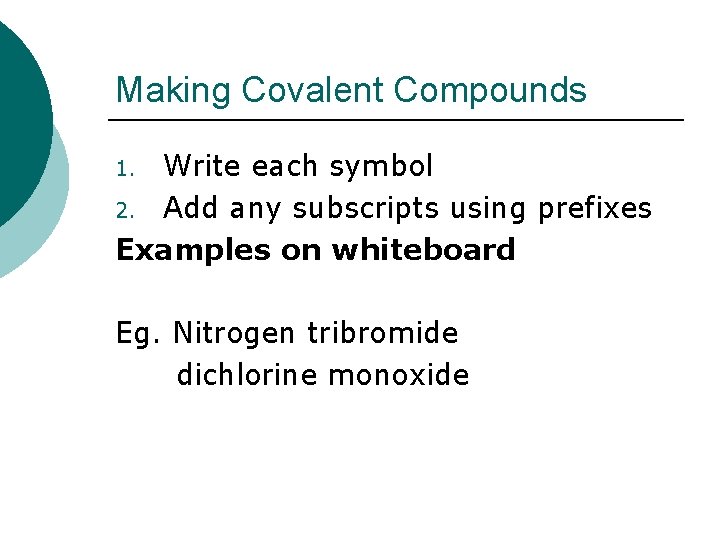
Making Covalent Compounds Write each symbol 2. Add any subscripts using prefixes Examples on whiteboard 1. Eg. Nitrogen tribromide dichlorine monoxide
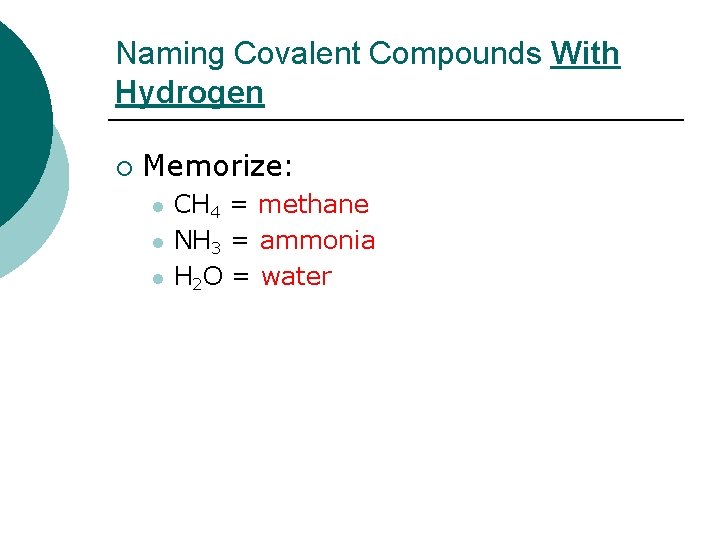
Naming Covalent Compounds With Hydrogen ¡ Memorize: l l l CH 4 = methane NH 3 = ammonia H 2 O = water





Naming Covalent Compounds With Hydrogen l l ¡ HF = hydrogen fluoride HCl = hydrogen chloride HBr = hydrogen bromide HI = hydrogen iodide But when dissolved in water they become acids: l l HF(aq) = hydrofluoric acid HCl(aq) = hydrochloric acid HBr(aq) = hydrobromic acid HI(aq) = hydroiodic acid
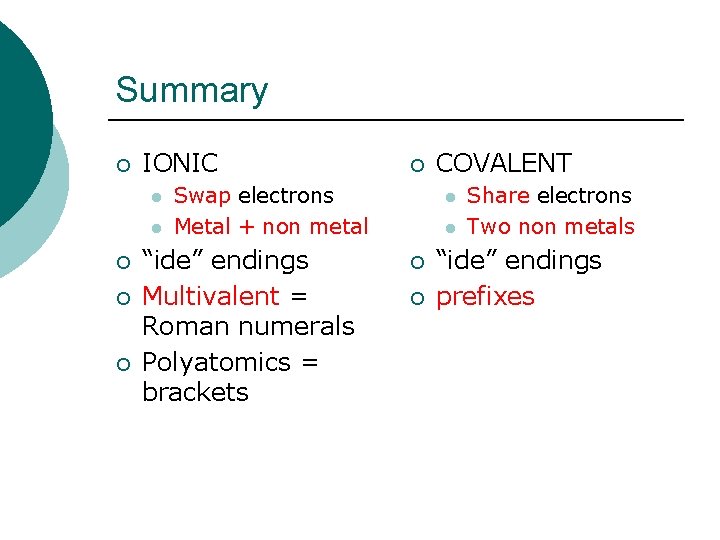
Summary ¡ IONIC l l ¡ ¡ Swap electrons Metal + non metal “ide” endings Multivalent = Roman numerals Polyatomics = brackets COVALENT l l ¡ ¡ Share electrons Two non metals “ide” endings prefixes

- Slides: 26