Science 10 Unit 1 Lesson 8 Balancing Equations

Science 10 Unit 1: Lesson 8 Balancing Equations

Objectives By the end of the lesson you should be able to: n balance a chemical reaction

Reactions n n n Whatever goes into a reaction, must come out. Atoms are REARRANGED, but are never CREATED or DESTROYED (this is called the Theory of Conservation of Mass). So we can balance the reaction by making sure there are the same number of atoms on both sides of the equation.

So what can we do? n You CANNOT change the subscript (sub means below…) n n n CH 2 You CAN multiply by placing a COEFFICIENT in front of the individual elements/compounds. So let’s count some atoms…. .
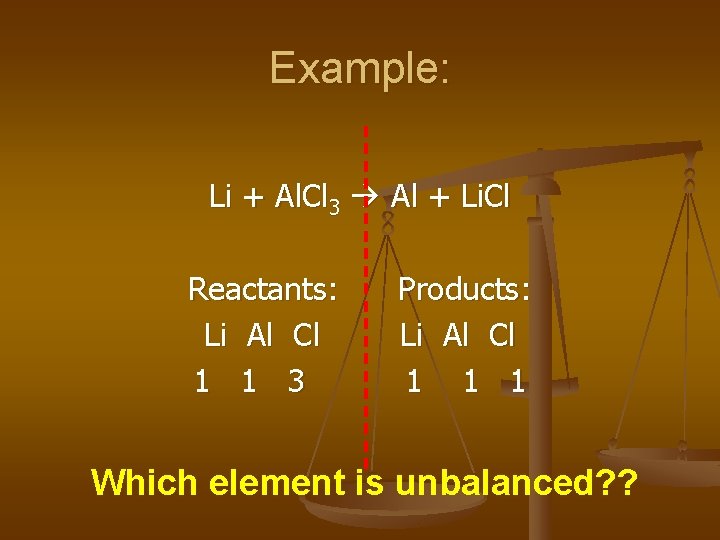
Example: Li + Al. Cl 3 Al + Li. Cl Reactants: Li Al Cl 1 1 3 Products: Li Al Cl 1 1 1 Which element is unbalanced? ?

Example: To balance this equation we have to make the Cl’s balanced on both sides How many more Cl’s do we need? 3 So we place a 3 infront of the Li. Cl molecule But that also means we have 3 Li’s So we need a 3 infront of the Li reactants Recount – is everybody balanced now? 3 Li + Al. Cl 3 Al + 3 Li. Cl

Steps: 1. 2. 3. 4. Count number of atoms on each side of equation Determine if there any that are unbalanced Make one change Recount atoms – if there any still unbalanced repeat step 3 – if all is balanced go on to the next question!

Your Turn! n n Use the booklet to practice balancing each type of reaction The booklet starts out easy then gets harder so don’t worry if you struggle near the end!
- Slides: 8